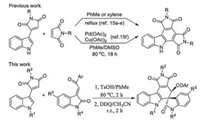
Among the various nitrogen-containing heterocyclic systems, carbazole is one of the most biologically important privileged heterocycle. The motif of carbazole exists widely in various naturally occurring alkaloids and pharmacologically active compounds and is featured in a large family of synthetic analogues exhibiting broad spectrum of important bioactivities, such as anti-tumor activity, anti-protein kinase C activity [1-3]. Additionally, the carbazole derivatives also have potential applications in organic light-emitting diodes and other functional materials [4]. Therefore, the development of efficient synthetic methodologies for functionalized carbazole derivatives have been attracted much attentions from organic and medical chemistry [5, 6]. Among the various elegant synthetic protocols, Diels-Alder reaction of active 2-vinylindolines or 3-vinylindolines with diverse dienophiles has become the most attractive strategy for the synthesis of many carbazole derivatives [7, 8]. On the other hand, spirooxindole represents the core structure of a wide variety of medicinal agents and natural products with broad biological activities ranging from antitumor, antimicrobial, anti-HIV and antipyretics agents [9]. As a consequence, considerable interest have been paid for the development of elegant synthetic methodologies for diverse spirooxindole derivatives [10]. The merge of carbazole with spirooxindole might result in the privileged heterocyclic system with interesting biological activities. The readily available and multifunctionalized 3-methyleneoxindoles are active dipolarophilic and dienophilic synthons and have been employed to react with various 2-vinyl and 3-vinylindolines to give functionalized spiro-carbazole-oxindole derivatives [11, 12]. In recent years, we have successfully developed several domino Diels-Alder reactions between the in situ generated active 2-vinyl or 3-vinylindolines with 3-methyleneoxindoles for synthesis of versatile spiro-carbazole-oxindole sysemes [13]. In this respect, 3-(indol-3-yl)maleimides can be regarded as one of the masked 3-vinylindolines [14]. Even through the C=C double bond connected to two electron-withdrawing carbonyl groups, the Diels-Alder of 3-(indol-3-yl)maleimides with common active 1, 3-dienophiles such as maleimides, cyclopent-2-enone, β-cis-cyanoacrylate at definte conditions have been reported in early years (Scheme 1) [15a-e]. Recently, Zhao and coworkers also sucessfully developed an effcient Pd(OAc)2 catalyzed Diels-Alder reaction of 3-(indol-3-yl)maleimides with maleimide for indolopyrrolocarbazoles [15f]. With our continuous aim of domino reactions for convenient assembly of various spirooxindoles [16, 17]. Herein, we wish to report the convenient synthesis of 4′, 6′-dihydrospiro[indoline-3, 5′-pyrrolo[3, 4-c]carbazoles] via one-pot two-step reaction of p-TsOH catalyzed Diels-Alder reaction of 3-(indol-3-yl)maleimides with 3-phenacylideneoxindoles and sequential dehydrogenation reactions.
|
Download:
|
| Scheme 1. Diels-Alder reaction of 3-(indol-3-yl)maleimides. | |
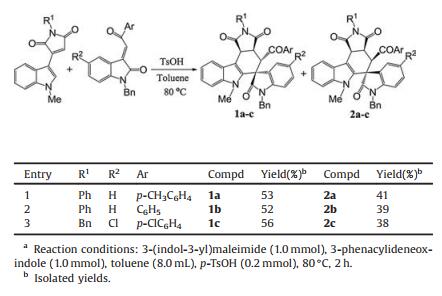
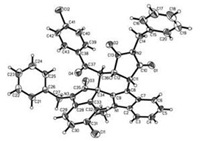
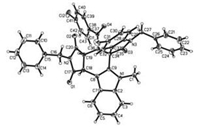
Initially, the reaction conditions was optimized with the reaction of 3-(1-methylindol-3-yl)-N-phenylmaleimide [16] and 3-phenacylideneoxindole as model reaction. When the reaction was carried out in refluxed in toluene without any catalyst, no reaction was observed after refluxing overnight with TLC monitoring. When a few amount of p-TsOH was added, the reaction proceeded smoothly to give the expected spiro products. When TfOH was used as catalyst, the reaction also gave the spiro compounds with some byproducts. After carefully examination, it was found that the best result was obtained by carrying the reaction in toluene at 80 ℃ with 20% mol of p-TsOH as catalyst for 2 h (Table 1). However, the reaction usually resulted in a mixture of cis/trans-tetrahydrospiro[indoline-3, 5′-pyrrolo[3, 4-c]carbazoles] 1a–1c and 2a–2c in nearly comparing yields. The cis/trans-isomers could be separated by column chromatography and were successfully characterized with various spectroscopy including 2D NMR spectra of the compounds 1c and 2c. The cis/trans-isomers clearly come from the endo- or exo-stereoselectivity of Diels-Alder reaction between the cyclic dienes and dienophiles. In order to elucidate the relative configuration of the cis/trans-isomers, the single crystal structures of the compounds 1c (Fig. 1, CCDC: 2016991) and 2c (Fig. 2, CCDC: 2016992) were successfully determined by X-ray diffraction method. From the Fig. 1, it can be seen that the fused motif of N-phenylsuccinimide exists on trans-position of the benzoyl group and the phenyl group in oxindole moiety in the trans-isomer 1c. In Fig. 2, the motif of N-phenylsuccinimide stands on cis-position of the benzoyl group and the phenyl group in oxindole moiety in the cis-isomer 2c. However, the benzoyl group and the phenyl group in oxindole moiety exists on cis-position in both cis-isomer 2c and trans-isomer 1c. Because the benzoyl group and the phenyl group in oxindole moiety originally exist on cis-position in the starting 3-phenacylideneoxindoles, the relative configuration of the two groups was retained in the reaction, which also indicated that an acid-catalyzed concerted Diels-Alder reaction process was involved in this reaction.
|
|
Table 1 Formation of cis/trans-tetrahydrospiro[indoline-3, 5′-pyrrolo[3, 4-c]carbazoles]a. |
|
Download:
|
| Fig. 1. Single crystal structure of the compound 1c. | |
|
Download:
|
| Fig. 2. Single crystal structure of the compound 2c. | |
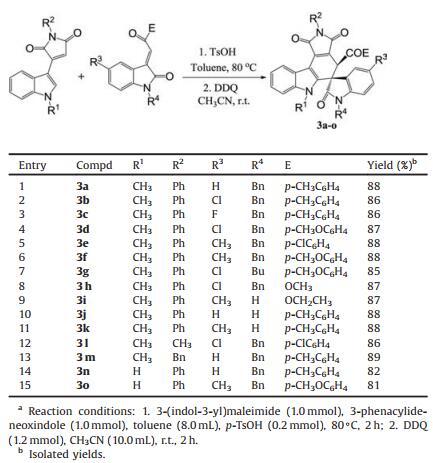
Because the above acid-catalyzed Diels-Alder reaction afforded a mixture of cis/trans-isomers in nearly comparable yields, which decreased the potential application of this synthetic protocol, we decided to convert them to 4′, 6′-dihydrospiro[indoline-3, 5′-pyrrolo[3, 4-c]carbazole] by dehydrogenation process. After the acid-catalyzed Diels-Alder reaction was finished, further oxidation reaction with DDQ was carried out in acetonitrile at room temperature for 2h. By using this one-pot two-step reaction process, the expected 4′, 6′-dihydrospiro[indoline-3, 5′-pyrrolo[3, 4-c]carbazoles] 3a-3o were successfully prepared in high yields. The results are summarized in Table 2. Various substituted 3-phenacylideneoxindoles reacted smoothly to give the expected products. The 3-(2-alkoxycarbonyl)methyleneoxindoles also gave the spiro compounds 3h and 3i in good yields. Besides normal 3-(1-methylindol-3-yl)pyrrole-2, 5-dione, it should be noticed that 3-(indol-3-yl)pyrrole-2, 5-dione with NH group can be successfully employed in the reaction to give the spiro compounds 3n and 3o in high yields. The structures of the obtained spiro compounds 3a-3o were fully characterized by various spectroscopies. The single crystal structures of the compounds 3a (Fig. 3, CCDC: 2016993), 3e (CCDC: 2016994), 3f (CCDC: 2016995), 3l–n (Figs. S1–S5 in Supporting information, CCDC: 2016996, CCDC: 2016997, CCDC: 2016998 for 3l, 3m and 3n, respectively) were successfully determined by X-ray diffraction. We were pleased to find that the same relative configuration were observed in these six single crystal structures, in which the scaffold of pyrrolo[3, 4-c]carbazole exist in nearly one plane, the benzoyl group and the phenyl group of the oxindole moiety stand incis-position as that of the cis/trans-isomers 1 and 2. Thus, we were pleased to find that the relative configurations of these two groups were retained in the one-pot two-step reaction.
|
|
Table 2 Synthesis of spiro[indoline-3, 5′-pyrrolo[3, 4-c]carbazoles] 3a-3oa. |

|
Download:
|
| Fig. 3. Single crystal structure of the spiro compound 3a. | |
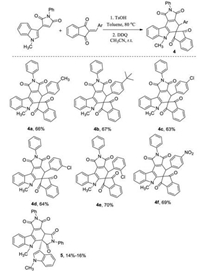
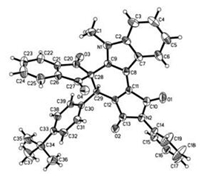
For developing the scope of this one-pot two-step reaction, 2-arylidene-1, 3-indanediones were employed to react with 3-(1-methylindol-3-yl)pyrrole-2, 5-dione under same reaction conditions. The results were summarized in Scheme 2. The expected dihydrospiro[indene-2, 5′-pyrrolo[3, 4-c]carbazoles] 4a–4f were successfully obtained in moderate to good yields. The substituents in 2-arylidene-1, 3-indanediones showed marginal effect on the yields. It should be pointed out that a byproduct 5 was isolated in lower yields in all cases, which were obviously come from the self-Diels-Alder reaction of 3-(1-methylindol-3-yl)-N-phenylmaleimide. The similar dimerization has been noticed in the previously reported reaction of 3-(1-indol-3-yl)-N-phenylmaleimides [14b]. The structures of the spiro compounds were characterized by IR, HRMS, 1H and 13C NMR spectra. Because there is only one possible diastereoisomer in the spiro compounds 4a-4f, the 1H NMR spectra clearly showed one set of absorption peaks for the characteristic groups. The single crystal structure of the spiro compound 4e (Fig. 4, CCDC: 2016999) and dimer 5 (Fig. S6 in Supporting information) were successfully determined.
|
Download:
|
| Scheme 2. Synthesis of spiro[indene-2, 50-pyrrolo[3, 4-c]carbazoles] 4a-4f. Reaction conditions: 1. 3-(indol-3-yl)maleimide (1.0 mmol), 2-arylidene-1, 3-indanedione (1.0 mmol), toluene (8.0 mL), p-TsOH (0.2 mmol), 80 ℃, 2 h; 2. DDQ (1.2 mmol), CH3CN (10.0 mL), r.t., 2 h. | |
|
Download:
|
| Fig. 4. Single crystal structure of the spiro compound 4e. | |
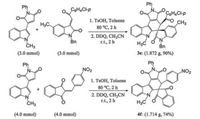
For demonstrating the synthetic values of this Diels-Alder reaction, the gram-scale reactions for the preparation of the spiro compounds 3e and 4f were carried out under standard reaction conditions (Scheme 3). We were pleased to find that the two spiro compounds 3e and 4f were successfully prepared in 90% and 74% yields, respectively, which were slightly higher than the previous yields in the microscale reactions.
|
Download:
|
| Scheme 3. Gram-scale synthesis of spiro compounds 3e and 4f. | |
In summary, we have investigated one-pot two-step of 3-(indol-3-yl)maleimides with 3-phenacylideneoxindoles or 2-arylidene-1, 3-indanediones. This reaction successfully provided an efficient synthetic protocol for the construction of 3a', 4′, 6′, 10c'-tetrahydro- and 4′, 6′-dihydro-spiro[indoline-3, 5′-pyrrolo[3, 4-c]carbazoles] as well as dihydrospiro[indene-2, 5′-pyrrolo[3, 4-c]carbazole]. The advantages of this simple reaction included using readily available reagents, good yields, high diastereoselectivity and molecular diversity. The reaction mechanism included Lewis acid catalyzed Diels-Alder reaction of electron-deficient diene with electron-deficient dienophiles and sequential oxidation reaction. The potential applications of this reaction for synthesis of complex heterocyclic spiro compounds might be significant.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21572196) and the Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2020.08.052.
| [1] |
(a) L.T. Shen, P.L. Shao, S. Ye, Adv. Synth. Catal. 353 (2011) 1943-1948; (b) T.Z. Li, Y. Jiang, Y.Q. Guan, et al., Chem. Commun. 50 (2014) 10790-10792; (c) T.P. Gao, J.B. Lin, X.Q. Hu, et al., Chem. Commun. 50 (2014) 8934-8936; (d) Y. Que, T. Li, C. Yu, et al., Org. Chem. 80 (2015) 3289-3294. |
| [2] |
(a) G.J. Mei, F. Shi, Chem. Commun. 54 (2018) 6607-6621; (b) G.J. Mei, D. Li, G.X. Zhou, et al., Chem. Commun. 53 (2017) 10030-10033; (c) J.L. Wu, B.X. Du, Y.C. Zhang, et al., Adv. Synth. Catal. 358 (2016) 2777-2790; (d) Q.N. Zhu, Y.C. Zhang, M.M. Xu, et al., J. Org. Chem. 81 (2016) 7898-7907. |
| [3] |
(a) K. Thevissen, A. Marchand, P. Chaltin, et al., Curr. Med. Chem. 16 (2009) 2205-2211; (b) R. Hesse, O. Kataeva, A.W. Schmidt, et al., Chem. Eur. J. 20 (2014) 9504-9509; (c) M.S. Shaikh, R. Karpoormath, N. Thapliyal, et al., Anticancer Agents Med. Chem. 15 (2015) 1049-1065; (d) S.P. Zhu, W.Y. Wang, K. Fang, et al., Chin. Chem. Lett. 25 (2014) 229-233. |
| [4] |
(a) H.J. Jiang, J. Sun, J.L. Zhang, Curr. Org. Chem. 16 (2012) 2014-2025; (b) K.T. Kamtekar, A.P. Monkman, M.R. Bryce, Adv. Mater. 22 (2010) 572-582; (c) M.K. Hong, M.K. Ravva, P. Winget, et al., Chem. Mater. 28 (2016) 5791-5798; (d) B.W. Li, X.A. Song, X. Jiang, et al., Chin. Chem. Lett. 31 (2020) 1188-1192; (e) Y. Xiong, J.J. Zeng, et al., Chin. Chem. Lett. 30 (2019) 592-596. |
| [5] |
(a) H.J. Knolker, R.R. Kethiri, Chem. Rev. 102 (2002) 4303-4427; (b) A.W. Schmidt, K.R. Reddy, H.J. Knlker, Chem. Rev. 112 (2012) 3193-3328; (c) S. Lancianesi, A. Palmieri, M. Petrini, Chem. Rev. 114 (2014) 7108-7149; (d) S.Z. Zhao, R.B. Andrade, J. Am. Chem. Soc. 135 (2013) 13334-13337; (e) T.L. Lan, H.J. Qin, W.T. Chen, et al., Chin. Chem. Lett. 31 (2020) 357-360. |
| [6] |
(a) V.P. Kumar, K.K. Gruner, O. Kataeva, et al., Angew. Chem. Int. Ed. 52 (2013) 11073-11077; (b) S.H. Cho, J. Yoon, S. Chang, J. Am. Chem. Soc. 133 (2011) 5996-6005; (c) A.C. Hernandez-Perez, S.K. Collins, Angew. Chem. Int. Ed. 52 (2013) 12696-12700; (d) H. Gao, Q.L. Xu, M. Yousufuddin, et al., Angew. Chem. Int. Ed. 53 (2014) 2701-2705. |
| [7] |
(a) C. Liu, X.Q. Han, X. Wang, et al., J. Am. Chem. Soc. 126 (2004) 3700-3701; (b) N. Kuroda, Y. Takah ashi, K. Yoshinaga, et al., Org. Lett. 8 (2006) 1843-1845; (c) F. Zhao, N. Li, Y.F. Zhu, et al., Org. Lett. 18 (2016) 1506-1509; (d) S.Z. Zhao, R.B. Andrade, J. Org. Chem. 82 (2017) 521-531. |
| [8] |
(a) C. Gioia, A. Hauville, L. Bernardi, et al., Angew. Chem. Int. Ed. 47 (2008) 9236-9239; (b) Y. Tao, F. Zhang, C.Y. Tang, et al., Asian J. Org. Chem. 3 (2014) 1292-1301; (c) L.J. Zhou, B. Xu, J.L. Zhang, Angew. Chem. Int. Ed. 54 (2015) 9092-9096; (d) J.W. Ren, Z.F. Zhou, J.A. Xiao, et al., Eur. J. Org. Chem. 7 (2016) 1264-1268. |
| [9] |
(a) Y.T. Yang, J.F. Zhu, G.C. Liao, et al., Med. Chem. 25 (2018) 2233-2244; (b) N. Ye, H.Y. Chen, E.A. Wold, et al., ACS Infect. Dis. 2 (2016) 382-392; (c) B. Yu, D.Q. Yu, H.M. Liu, Eur. J. Med. Chem. 97 (2015) 673-698; (d) B. Yu, Z.Q. Yu, P.P. Qi, et al., Eur. J. Med. Chem. 95 (2015) 35-40. |
| [10] |
(a) G.S. Singh, Z.Y. Desta, Chem. Rev. 112 (2012) 6104-6155; (b) L. Hong, R. Wang, Adv. Synth. Catal. 355 (2013) 1023-1052; (c) Y. Liu, H. Wang, J. Wan, Asian J. Org. Chem. 2 (2013) 374-386; (d) Z. Liu, N. Li, X. Huang, et al., Tetrahedron. 70 (2014) 2406-2415. |
| [11] |
(a) B. Tan, G. Hernandez-Torres, C.F. Barbas, J. Amer. Chem. Soc. 133 (2011) 12354-12357; (b) H.F. Zheng, P. He, Y.B. Liu, et al., Chem. Commun. 50 (2014) 8794-8796; (c) P. Sharma, N.P. Kumar, N.H. Krishna, et al., Org. Chem. Front. 3 (2016) 1503-1508; (d) Z.H. You, Y.H. Chen, Y. Tang, et al., Org. Lett. 20 (2018) 6682-6686. |
| [12] |
(a) Y.K. Liu, M. Nappi, E. Arceo, et al., J. Am. Chem. Soc. 133 (2011) 15212-15218; (b) Y. Wang, M.S. Tu, L. Yin, et al., J. Org. Chem. 80 (2015) 3223-3232; (c) J.W. Ren, J. Wang, J.A. Xiao, et al., J. Org. Chem. 82 (2017) 6441-6449; (d) L.J. Huang, J. Weng, S. Wang, et al., Adv. Synth. Catal. 357 (2015) 993-1003. |
| [13] |
(a) R.Y. Yang, J. Sun, Y. Tao, et al., J. Org. Chem. 82 (2017) 13277-13287; (b) R.Y. Yang, J. Sun, Q. Sun, C.G. Yan, J. Org. Chem. 83 (2018) 5909-5919; (c) D.Q. Wang, J. Sun, C.G. Yan, Chemistryselect 4 (2019) 10550-10554; (d) J. Sun, R.Y. Yang, S.C. Zhan, et al., ChemistrySelect 4 (2019) 10100-10103; (e) R. Ye, C.G. Yan, Eur. J. Org. Chem (2019) 5882-5886; (f) S.C. Zhan, J. Sun, R.Z. Liu, et al., Org. Biomol. Chem. 18 (2020) 163-168. |
| [14] |
(a) Y.L. An, Z.Y. Shao, J. Chen, et al., Synthesis 45 (2013) 2719-2726; (b) E. Pereira, A. Youssef, M. El-Ghozzi, et al., Tetrahedron Lett. 55 (2014) 834-837. |
| [15] |
(a) H. Henon, S. Messaoudi, B. Hugon, et al., Tetrahedron 61 (2005) 5599-5614; (b) E. Conchon, F. Anizon, B. Aboab, et al., Bioorg. Med. Chem. 16 (2008) 4419-4430; (c) B. Hugon, B. Pfeiffer, P. Renard, et al., Tetrahedron Lett. 44 (2003) 3935-3937; (d) E. Conchon, F. Anizon, B. Aboa, et al., J. Med. Chem. 50 (2007) 4669-4680; (e) H. Hénon, F. Anizon, N. Kucharczyk, et al., Synthesis (2006) 711-715; (f) Y.L. An, Z.H. Yang, H.H. Zhang, et al., Org. Lett. 18 (2016) 152-155. |
| [16] |
(a) J. Sun, Y. Sun, H. Gong, et al., Org. Lett. 14 (2012) 5172-5175; (b) J. Sun, Y.J. Xie, C.G. Yan, J. Org. Chem. 78 (2013) 8354-8365; (c) H. Gao, J. Sun, C.G. Yan, J. Org. Chem. 79 (2014) 4131-4136; (d) Y. Han, Y.J. Sheng, C.G. Yan, Org. Lett. 16 (2014) 2654-2657; (e) J. Sun, L. Chen, H. Gong, et al., Org. Biomol. Chem. 13 (2015) 5905-5917; (f) L. Chen, J. Sun, J. Xie, et al., Org. Biomol. Chem. 14 (2016) 6497-6507. |
| [17] |
(a) R.G. Shi, X.H. Wang, C.G. Yan, et al., Chem. Commun. 52 (2016) 6280-6283; (b) J. Cao, J. Sun, C.G. Yan, Org. Biomol. Chem. 16 (2018) 4170-4175; (c) R.Z. Liu, R.G. Shi, J. Sun, et al., Org. Chem. Front. 4 (2017) 354-357; (d) J. Sun, Y. Zhang, R.G. Shi, et al., Org. Biomol. Chem. 17 (2019) 3978-3983; (e) J. Cao, F. Yang, J. Sun, et al., J. Org. Chem. 84 (2019) 622-635. |
 2021, Vol. 32
2021, Vol. 32