b The Key Laboratory of Functional Molecular Solids, Ministry of Education, School of Chemistry and Materials Science, Anhui Normal University, Wuhu 241002, China
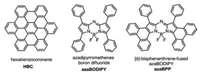
Organic compounds with polycyclic aromatic hydrocarbons generally show superior optoelectronic and self-assembling properties due to the π-π interaction between molecules [1-3]. The self-assembly process is depending on molecular structure, which could be manipulated through molecular structure engineering [4-6]. This has been well demonstrated by the extensively studied hexabenzocoronene (HBC, Fig. 1) and their heteroatom-doped derivatives system, such as forming one dimensional (1D) micro- or nanowires [7, 8], nanotubes [9-11], two dimensional (2D) nanosheets [12, 13], which show great potential in electronic devices, such as organic field effect transistors and photodetectors [14, 15].
|
Download:
|
| Fig. 1. Core structures of hexabenzocoronene (HBC), azadipyrromethenes boron difluoride (azaBODIPY) and [b]-bisphenanthrene-fused azaBODIPYs (azaBPP). | |
Boron dipyrromethene dyes (BODIPYs) and their derivatives, as an increasingly valuable class of fluorophores, have been developed through various modification approaches [16-20]. Replacing the meso carbon atom of BODIPY by nitrogen atom to form azaBODIPY dyes (Fig. 1), which were first reported by O'Shea et al. have received extensive research interests lately due to their excellent stabilities (associated with their rigid structures) and their remarkable optic and electronic properties, especially the tunable deep-red to NIR absorptions/emissions [21-23, 24]. Indeed, their highly electron-deficient conjugation core skeletons with large dipole moments are ideal features for the promising organic electronics and self-assembly properties [25-27]. For example, the amphiphilic azaBODIPY molecule can self-assemble into globular nanoparticles and elongated nanorods by J-type aggregation mode [28-30]. The azaBODIPY molecule introduced with phosphoric acid can self-assemble to form nanosphere shells [31]. However, due to the limitation of the synthesis, there are few reports about the π-extended conjugated azaBODIPY. Until recently, we reported [a]- and [b]-bisphenanthrene-fused azaBODIPYs (azaBPP, Fig. 1) through oxidative aromatic coupling and palladium catalyst C–H activation reaction [32-35]. These π-extended azaBODIPY molecules not only exhibit excellent near infrared optical activity and interesting electronics properties, but also provide an opportunity to produce discrete (isolable) nanostructures through self-assembly. Despite the effects toward controlling and tuning the morphology of related porphyrinoids [36-38] and the above HBC molecules, the facile synthesis of well-defined, large nanostructures with NIR absorption over 800 nm, which have promising applications in important areas such as sensors, phototheranostic agents, and solar power, is still limited.
Herein, we synthesized a [b]-bisphenanthrene-fused azaBODIPY containing four long hydrophobic chains on the periphery (azaBPP-12C). Combined the π-π and side chain interactions, azaBPP-12C can be self-assembled into one-dimensional microwires through the solution process. The microwires were characterized using scanning electron microscope (SEM) and X-ray diffraction (XRD), the length can reach hundreds of microns. The aggregation behavior of azaBPP-12C in solution was confirmed as H-type aggregation by absorption spectroscopy.
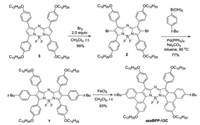
[b]-PhenanthroazaBODIPY azaBPP-12C with four side chain was synthesized in Scheme 1. 1, 3, 5, 7-Tetra-(4-dodecyloxyphenyl) azaBODIPY 3, as the key starting material with long chain installed initially, was generated in an overall yield of 30% in three steps (Scheme S1 in Supporting information) from commercially available 4-dodecyloxybenzaldehyde and 4-dodecyloxyacetophenone. After bromination, the dibromoazaBODIPY 2 was then applied in the Suzuki coupling reaction with 4-tert-butylphenylboronic acid to afford the corresponding azaBODIPY 1 in 77% yield (Scheme 1). The subsequent oxidative ring-fusion reaction with 10 equiv. of FeCl3 in CH2Cl2/CH3NO2 at room temperature afforded target product azaBPP-12C successfully in high yield.
|
Download:
|
| Scheme 1. Synthesis of [b]-Bisphenanthrene-Fused AzaBODIPYs azaBPP-12C. | |
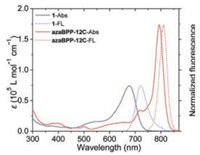
The UV–vis absorption as well as fluorescence spectra of 1 and azaBPP-12C in chloroform (Fig. 2) show all of the typical spectral features of substituted azaBODIPY and [b]-phenanthroazaBODIPYs reported in the literatures [33, 34]. Compound 1 exhibits absorption band around 580−730 nm, with a maximum peak at 657 nm and the corresponding emission maximum at 683 nm (ϕ=0.25). After the cyclization, the absorption bands of azaBPP-12C undergo significant red shifts to 700−830 nm, with the maximum absorption peak at 790 nm. The molar extinction coeffient value of the ring-fusion compounds azaBPP-12C reaches 173, 800 L mol−1 cm−1, which is double greater than that of 1 (74, 100L mol−1 cm−1).
|
Download:
|
| Fig. 2. Absorption (Abs) and fluorescence (FL) spectra of compounds 1 and azaBPP-12C in chloroform (5×10-6 mol/L). | |
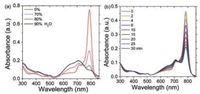
The self-assembly behaviors of azaBPP-12C in solution was monitored by the absorption spectral measurement. Initially, azaBPP-12C keeps the original characteristic absorption peak in THF (Fig. 3a). Progressively increasing the volume fraction of water, as a poor solvent, in THF does not affect its spectrum until the solution consists of 70% water. The intensity of the main absorption band was decreased greatly, while the absorption at shorter wavelength became enhanced. No red-shifted absorption band was observed, which indicated that an H-aggregation of azaBPP-12C was formed during the aggregation process [39, 40]. Continuing to increase the fraction of water to 90%, the absorption intensity of the short wavelength was increased with a slight lift up around 850 nm, which may be due to the extended aggregate states. The same aggregation process was also detected in ethanol solution (Fig. 3b). As time goes on, the absorption below 790 nm was gradually decreased, while the absorption at shorter wavelength became enhanced. These results indicate the dominant role of π-π interaction between the molecules of azaBPP-12C.
|
Download:
|
| Fig. 3. Spectroscopic changes observed during aggregation for azaBPP-12C: (a) Evolution of the absorption spectrum as the water content increased from 0 to 90% (v/v) in THF/water mixture (4×10-6 mol/L). (b) Evolution of the absorption spectrum in ethanol at different aging times: from 0 to 30 min (3×10-6 mol/L). | |
The face-to-face arrangement of π-π interaction between the molecules typically guarantees the formation of 1D materials [41]. The morphology of the aggregates formed was then examined by scanning electron microscopy (SEM). Initially, we used the method of poor solvent mixing to prepare the aggregates. At room temperature, methanol continuously mixed into the chloroform solution of azaBPP-12C. As the poor solvent methanol continuously increased, the solubility of the mixed solvent to azaBPP-12C was decreased. Under π-π intermolecular interaction and alkyl chain interaction, one-dimensional nanowires were indeed gradually formed (Fig. S1 in Supporting information). However, the speed of poor solvent mixing may be too fast, the nanowires formed by this method have great defects.
Finally, the well-ordered 1D nanowires of azaBPP-12C were precipitated by cooling hot saturated solution. A suspension of azaBPP-12C in MeOH/THF (ratio: 1/2) was heated to form a homogeneous solution. Then the solution was cooled to room temperature slowly, allowing the molecules to self-assemble. The nanowires obtained in MeOH/THF mixed solvent are around 1 μm in width and hundreds of micrometers in length (Figs. 4a-b and Fig. S2 in Supporting information). The microwires were further characterized by the X-ray diffraction analysis. The first and second diffraction peaks (d-spacing=21.8 and 17.0 Å) are assigned to the edge-to-edge distances between adjacent azaBPP-12C molecules at the long and short axis directions (Fig. 4g). The broad diffuse scattering was observed in the middle-angle region (d-spacing = 7.1 Å), which is characteristic of the liquid-like order of the hydrocarbon chains. Combined with absorption spectral results, these data indicated that the molecules adopt face to face arrangement. A one-dimensional columnar arrangement model was proposed (Fig. 4h). The diffraction peaks in the high-angle region (d-spacing=4.3 and 4.4 Å) were assigned to the periodic correlations of the cores along the columns.
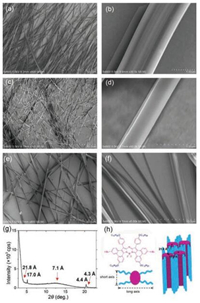
|
Download:
|
| Fig. 4. (a, b) SEM images (a, scale bar: 50 mm; b, scale bar: 1 mm) and (g) XRD pattern of the nanowires grown from 1:2 MeOH: THF solvent. (c, d) SEM images of the nanowires grown from n-hexane (scale bar: c, 50 mm; d, 1 mm). (e. f) SEM images of the nanowires grown from 1, 4-dioxane (scale bar: e, 50 mm; f, 10 mm). (h) The proposed model of the columnar stacking structure for azaBPP-12C. | |
When the solvent is changed from MeOH/THF mixed solvent to n-hexane or 1, 4-dioxane, respectively, similar nanowires were also observed in both solvents using the same method (Figs. 4c-f). However, the nanowires obtained in hexane are much thinner, with a width around 500 nm, while the nanowires obtained in 1, 4-dioxane are thicker and more rigid, with a width around 2 μm. In addition, these nanowires obtained in both 1, 4-dioxane and n-hexane look softer compared to those obtained in MeOH/THF mixed solvent. These changes in the morphology of self-assembled nanoscale aggregates indicated the solvent effect on the formation of self-assembled nanostructures of azaBPP-12C molecules.
In summary, a [b]-bisphenanthrene-fused azaBODIPY with four side chain was prepared through the de novo synthesis method. This π-extended azaBODIPY shows strong, red-shifted absorption and emission in NIR region with maximum peaks up to 793 nm and 810 nm, respectively. Due to the enhanced π-π interaction, azaBPP-12C adopted H-aggregation during the aggregation. The ordered 1D microwires are also obtained by solution process and characterized using SEM and XRD, which are promising materials for the application in organic devices.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work is supported by the National Nature Science Foundation of China (Nos. 21672006, 21672007, 21871006), Laboratorial Open Fund of Ministry of Education of Anhui Normal University (No. FMS201914), and Natural Science Research Fund of BengBu Medical College (No. BYKY2019005ZD).
Appendix A. Supplementary dataSupplementar ymaterial related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2020.08.047.
| [1] |
M. Stępień, E. Gońka, M. Żyłaa, N. Sprutta, Chem. Rev. 117 (2017) 3479-3716. DOI:10.1021/acs.chemrev.6b00076 |
| [2] |
X. Luo, J. Li, J. Zhao, et al., Chin. Chem. Lett. 30 (2019) 839-846. DOI:10.1016/j.cclet.2019.03.012 |
| [3] |
Q. Fan, W. Ni, L. Chen, G.G. Gurzadyan, Y. Xiao, Chin. Chem. Lett. 31 (2020) 2965-2969. DOI:10.1016/j.cclet.2020.06.018 |
| [4] |
X. Zhang, Q. Wang, L. Wu, et al., J. Phys. Chem. B 114 (2010) 1233-1240. |
| [5] |
M. Al Kobaisi, S.V. Bhosale, K. Latham, A.M. Raynor, S.V. Bhosale, Chem. Rev. 116 (2016) 11685-11796. DOI:10.1021/acs.chemrev.6b00160 |
| [6] |
Z. Li, S. Chen, C. Gao, et al., J. Am. Chem. Soc. 141 (2019) 19448-19457. DOI:10.1021/jacs.9b10505 |
| [7] |
M. Kastler, W. Pisula, D. Wasserfallen, T. Pakula, K. Müllen, J. Am. Chem. Soc. 127 (2005) 4286-4296. DOI:10.1021/ja0430696 |
| [8] |
X.Y. Wang, F.D. Zhuang, R.B. Wang, et al., J. Am. Chem. Soc. 136 (2014) 3764-3767. DOI:10.1021/ja500117z |
| [9] |
W. Jin, Y. Yamamoto, T. Fukushima, et al., J. Am. Chem. Soc. 130 (2008) 9434-9440. DOI:10.1021/ja801179e |
| [10] |
W. Zhang, W. Jin, T. Fukushima, N. Ishii, T. Aida, J. Am. Chem. Soc. 135 (2013) 114-117. DOI:10.1021/ja311738m |
| [11] |
Y. Liu, W. Zeng, X. Li, W. Jin, D. Zhang, Dye. Pigment. 163 (2019) 553-558. DOI:10.1016/j.dyepig.2018.12.047 |
| [12] |
J. Luo, X. Xu, R. Miao, Q. Miao, J. Am. Chem. Soc. 134 (2012) 13796-13803. DOI:10.1021/ja3054354 |
| [13] |
Y. Zhou, M.Y. Zhang, K.H. Gu, et al., Asian J. Org. Chem. 4 (2015) 746-755. DOI:10.1002/ajoc.201500131 |
| [14] |
T. Aida, E.W. Meijer, S.I. Stupp, Science 335 (2012) 813-817. DOI:10.1126/science.1205962 |
| [15] |
T. Shimizu, W. Ding, N. Kameta, Chem. Rev. 120 (2020) 2347-2407. DOI:10.1021/acs.chemrev.9b00509 |
| [16] |
Q. Wang, X. Wei, C. Li, Y. Xie, Dye. Pigment. 148 (2018) 212-218. DOI:10.1016/j.dyepig.2017.09.020 |
| [17] |
F. Ma, L. Zhou, Q. Liu, C. Li, Y. Xie, Org. Lett. 21 (2019) 733-736. DOI:10.1021/acs.orglett.8b03954 |
| [18] |
G. Su, K. Zhang, F. Sha, et al., Dye. Pigment. 180 (2020) 108504. DOI:10.1016/j.dyepig.2020.108504 |
| [19] |
W. Sheng, F. Lv, B. Tang, E. Hao, L. Jiao, Chin. Chem. Lett. 30 (2019) 1825-1833. DOI:10.1016/j.cclet.2019.08.004 |
| [20] |
D. Wang, X. Guo, H. Wu, et al., J. Org. Chem. 85 (2020) 8360-8370. DOI:10.1021/acs.joc.0c00620 |
| [21] |
Y. Ge, D.F. O'Shea, Chem. Soc. Rev. 45 (2016) 3846-3864. DOI:10.1039/C6CS00200E |
| [22] |
K. Burgess, A. Loudet, Chem. Rev. 107 (2007) 4891-4832. DOI:10.1021/cr078381n |
| [23] |
H. Lu, J. Mack, Y. Yang, Z. Shen, Chem. Soc. Rev. 43 (2014) 4778-4823. DOI:10.1039/C4CS00030G |
| [24] |
Z. Wang, C. Cheng, Z. Kang, et al., J. Org. Chem. 84 (2019) 2732-2740. DOI:10.1021/acs.joc.8b03145 |
| [25] |
H. Wang, Y. Zhang, Y. Chen, et al., Angew. Chem. Int. Ed. 59 (2020) 5185-5192. DOI:10.1002/anie.201914966 |
| [26] |
Y. Liu, N. Song, Z. Li, L. Chen, Z. Xie, Dye. Pigment. 160 (2019) 71-78. DOI:10.1016/j.dyepig.2018.07.034 |
| [27] |
C. Liu, W. Ding, Y. Liu, H. Zhao, X. Cheng, New J. Chem. 44 (2020) 102-109. DOI:10.1039/C9NJ04755G |
| [28] |
Z. Chen, Y. Liu, W. Wagner, et al., Angew. Chem. Int. Ed. 56 (2017) 5729-5733. DOI:10.1002/anie.201701788 |
| [29] |
Y. liu, Y. Zhang, F. Fennel, et al., Chem. Eur. J. 24 (2018) 16388-16394. DOI:10.1002/chem.201803336 |
| [30] |
Y. Chen, X.H. Zhang, D.B. Cheng, et al., ACS Nano 14 (2020) 3640-3650. DOI:10.1021/acsnano.0c00118 |
| [31] |
M.H.Y. Cheng, K.M. Harmatys, D.M. Charron, J. Chen, G. Zheng, Angew. Chem. Int. Ed. 58 (2019) 13394-13399. DOI:10.1002/anie.201907754 |
| [32] |
J. Cui, W. Sheng, Q. Wu, et al., Chem. Asian J. 12 (2017) 2486-2493. DOI:10.1002/asia.201700876 |
| [33] |
W. Sheng, J. Cui, Z. Ruan, et al., J. Org. Chem. 82 (2017) 10341-10349. DOI:10.1021/acs.joc.7b01803 |
| [34] |
W. Sheng, Y.Q. Zheng, Q. Wu, et al., Org. Lett. 19 (2017) 2893-2896. DOI:10.1021/acs.orglett.7b01133 |
| [35] |
W. Sheng, Y. Wu, C. Yu, et al., Org. Lett. 20 (2018) 2620-2623. DOI:10.1021/acs.orglett.8b00820 |
| [36] |
Y. Gao, X. Zhang, C. Ma, X. Li, J. Jiang, J. Am. Chem. Soc. 130 (2008) 17044-17052. DOI:10.1021/ja8067337 |
| [37] |
Y. Gao, Y. Chen, R. Li, et al., Chem. Eur. J. 15 (2009) 13241-13252. DOI:10.1002/chem.200901722 |
| [38] |
P. Guo, P. Chen, M. Liu, Langmuir 28 (2012) 15482-15490. DOI:10.1021/la3033594 |
| [39] |
F. Würthner, T.E. Kaiser, C.R. Saha-Möller, Angew. Chem. Int. Ed. 50 (2011) 3376-3410. DOI:10.1002/anie.201002307 |
| [40] |
T. Li, J. Benduhn, Z. Qia, et al., J. Phys. Chem. Lett. 10 (2019) 2684-2691. DOI:10.1021/acs.jpclett.9b01222 |
| [41] |
Y. Guo, L. Xu, H. Liu, et al., Adv. Mater. 27 (2015) 985-1013. DOI:10.1002/adma.201403846 |
 2021, Vol. 32
2021, Vol. 32 

