b MOE Key Laboratory of Organic Optoelectronics and, Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing 100084, China
High-performance organic semiconductors have received widespread attention as the key components of various flexible devices such as organic field-effect transistors, light-emitting diodes, organic solar cells, sensors [1-6]. Aside from chemically synthesizing new molecules, appropriate regulation of the existing materials is an environmental-friendly and cost-effective way to achieve excellent properties, such as high mobility, piezochromic phenomenon and strong luminescence [7-11]. Due to the great flexibility of organic materials, applying mechanical force as a simple method could continuously regulate the performance of organic photoelectric materials [12].
Recently, significant performance which could boost on charge transport were observed by compressing organic semiconductors, such as TIPS-pentacene and single crystal rubrene [13, 14]. Besides, anomalous pressure effects, anisotropic or nonmonotonic phenomenon were also found [15, 16]. Limited to experimental testing methods, the mechanism of the pressure-regulation remains controversial, and is urgent to be explored theoretically. At the aspect of fluorescence emission behaviors, generally, enhancedπ-π interactions under pressure may cause fluorescence quenching in H-aggregates [17]. However, the suppression of intramolecular rotation and vibration under pressure would attenuate the excited state non-radiation process and promote pressure-induced emission enhancement (PIEE) phenomenon [18-21]. With the diamond anvil cell, more and more PIEE molecules have been found recently [22-24]. Although, it could be partly explained by the suppression of intramolecular rotation and vibration, as a completely new field, more theoretical researches should be carried out to understand it more thoroughly. By now, there are some individual works investigating the pressure effect on charge transport or optical properties of organic semiconductors. However, as we know, few works have studied the pressure effect on the two properties simultaneously. Therefore, it is essential to explore the influence of pressure on these two aspects at the same time, which will provide momentous guidance for the regulation of material performance.
In this work, we choose a high mobility emissive organic semiconductor, 2, 6-diphenylanthracene (DPA), Fig. 1 [25] and investigate the pressure-depended charge transport and luminescence properties by theoretical calculation. The dispersion-corrected density functional theory and hybrid quantum mechanics/molecular mechanics (QM/MM) method [26, 27] were used to get the cell parameters, electronic structures as well as vibration properties under pressure. Based on them, we then calculated the charge transport rates and mobilities with the quantum nuclear tunneling model. The thermal vibration correlation function was adopted to calculate fluorescence absorption/emission spectra and radiative/nonradiative decay rates [19, 28].
|
Download:
|
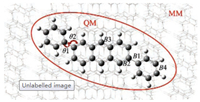
| Fig. 1. Molecular structure of DPA with marked bond length (B1, B2, B3, B4), dihedral angle (θ1) and bond angle (θ2) in QM/MM model. | |
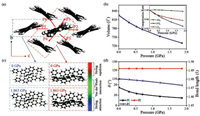
The cell parameters and atom positions of DPA crystals at different pressures were optimized and the calculated cell volume decreased monotonically from 825.15Å3 to 730.04Å3 with the extra pressure elevating to 1.863 GPa. However, the compression rates of cell parameters along different directions are distinct. FromFig. 2, b-axis, the most pressure-sensitive axis, reduced by 0.521Å, whilea-axis was almost unchanged. The difference could be understood as the planarization of the molecules on the bc-plane.
|
Download:
|
| Fig. 2. (a) Molecular packing modes of DPA single crystals. (b) The unit cell volume of DPA at different pressures. The insert figure shows the different compression rate of a-, b- and c-axis at different pressures. (c) Intermolecular interactions analyzed by RDG method at 0 GPa and 1.863 GPa. (d) Bond angle, dihedral angle and bond length of DPA at different pressures. | |
We then investigated the intermolecular interactions and intramolecular geometric changes at various pressures. When exploring the interactions between adjacent molecules, reduced density gradient (RDG) analysis [19] is adopted. From Fig. 2c. the space of Van der Waals interaction enlarged in both "face-to-face" and "face-to-edge" situation, indicating the enhanced C-H⋯π and π-π interactions at higher pressures. Hirshfeld surface was also introduced to describe the adjacent molecules with enhanced interactions (Fig. S1 in Supporting information). Typical dihedral angle (θ1), bond angle (θ2), and bond lengths (B1, B2, B3, B4) were chosen to explore the change of molecular structure with pressure. From Fig. 2d, the dihedral angle (θ1) between anthracene and phenyl ring is significantly reduced, making the whole molecule more planar. While the bond angle (θ2) of the anthracene ring is almost unchanged within the pressure range. And the bond length undergoes a slight decrease under the relatively high pressure.
To find out the pressure-depended charge transport properties, we calculated hole mobilities at different pressures. The results show a monotonous increase from 8.15 cm2V−1 s−1 to 15.72 cm2V−1 s−1, nearly twofold change (Fig. 3a). And the mobilities also maintain certain anisotropic and the maximum charge mobility occurs along the b-axis, owing to the dense packing and stronger interaction. To understand the mechanism of pressure effects on mobilities, the contributions of transfer integrals (V) and reorganization energies (λ) were further investigated. Transfer integral represents the degree of overlap between adjacent molecules, which is positively correlated with mobility (Eq. S1 in Supporting information). The reorganization energy, on the other hand, measures the coupling strength of electron-phonon interaction.

|
Download:
|
| Fig. 3. (a) The maximum mobility at different pressures and the inset picture shows hole transfer integral and adjacent molecular distance of P1 direction at different pressures. Projection of the total reorganization energy λ onto the (b) dihedral angle, (c) bond angle and bond length of DPA crystals at different pressures. | |
From the aspect of transfer integrals, extra pressure could induce huge transfer integrals. And meanwhile, the pressure response of transfer integrals has the path-dependence characteristic, because of different stacking modes [29]. As shown in Fig. 2, there are six effective transfer pathways between the central molecule and six neighbor molecules. Because of the symmetry, the transport paths along P1, P3, P4, P6 directions are equivalent. And there is also no difference between the P2 and P5 directions. It is easy to find the transfer integrals in both directions increase with pressure (from 56.58 meV to 83.62 meV for P1, and from 17.15 meV to 30.79 meV for P2, Table S2 in Supporting information), which is mainly due to the gradual decrease in the distance, around 0.3 Å, between corresponding dimer, making more effective electronic couplings.
The pressure-depended reorganization energies decrease from 151.89 meV to 145.89 meV with the pressure reaching 1.863GPa. To understand the behavior of reorganization energy changes, we projected them onto the different features of DPA molecular, bond angle, dihedral angle and bond length. In Fig. 3, it is obvious that all kinds of reorganization energies decreased with compression. For the dihedral angle, with the increase of pressure, the reorganization energies drop quickly and then maintain a slower rate of decrease, consistent well with the variation of dihedral angle discussed before. It decreased by nearly 65%, from 6.14 meV to 2.19 meV at 1.86GPa, which can be attributed to the obvious suppression of out-of-plane rotations of DPA in the selected pressure range. In contrast, the contributions of bond lengths and bond angles remain almost unchanged at lower pressures and drop at higher pressures. The Huang-Rhys factor and reorganization energy at different pressures are shown in Fig. S2 (Supporting information). At low pressures, the modes, within the lower frequency region, associated with the rotation of the dihedral angle are significantly inhibited. And with the pressure increase continuously, the contribution of stretching vibration of bond length and bending vibration of bond angle also started working in high-frequency region. Noticeably, the strict stacking and electrostatic effect from surrounding molecules are also important when investigating the vibration properties of DPA crystals. The calculated reorganization energy of individual DPA molecule is 172.10 meV, 20.21 meV more than of embedded DPA molecule in QM/MM model. To summarize, the increasing charge mobilities can be contributed by the much larger transfer integral as well as the smaller reorganization energy under pressure.
When considering the photophysical properties, there are two possible effects of pressure on fluorescence emission. One is the fluorescence quenching [19] with the increase of exciton coupling effects, another is the pressure-induced emission enhancement caused by the suppression of non-radiative decay process [8, 30]. At the same time, pressure may also cause a piezochromic phenomenon [12]. We firstly selected theJ/λe criterion, proposed by previous researchers [31], to measure the effect of exciton coupling on compressed DPA in our study.λe is reorganization energy in the excited state and J refers to the strength of the exciton coupling effect. Li and colleagues studied a series of organic optoelectronic materials and found their photophysical properties are mainly determined by the competition between the reorganization energy and exciton coupling strength in aggregates. When J/λ > 0.17, the strong exciton coupling would induce fluorescence quenching of 0−0 emission peak. While in many aggregation induced emission molecules, where J/λ < 0.17, the reorganization energy dominates and the exciton coupling effect could be negligible. Fig. 4a gives the changes of J and λe under different pressures. The results show that J increases slightly with pressure while λe decreases. When pressure is as high as 1.863GPa, the biggestJ/λe value is 0.103, indicating the exciton effect could be ignored in our system. And we no longer consider it in the subsequent calculations. Followed, we calculated and analyzed the electronic properties and fluorescence spectrums of DPA. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) are distributed throughout the whole molecule, indicating a typical local excitation characteristic and they are almost independent of pressure, detailed information can be found in Table S4 and Fig. S3 (Supporting information). The vibrationally resolved absorption and emission spectrums are presented in Figs. 4b and c. In the emission spectrum, there is only a slight red shift under low pressure, which could be ascribed to the planarization of the conformation at low pressure [17, 32].

|
Download:
|
| Fig. 4. (a) Reorganization energies λe, exciton couplings J and the ratio J/λe of DPA in aggregates at different pressures. Vibrationally resolved (b) absorption and (c) emission spectra at different pressures. The insets show the maximum absorption/emission wavelength at different pressures. | |
We then calculated fluorescence quantum yield () under pressure. The calculated, and are presented in Table S2 (Supporting information). With the increase of applied external pressure, the oscillator strength increases gradually and undergoes a slight increase. While shows an ignorable decrease, leading to no obvious change of. The non-radiative decay process is the key factor in understanding some aggregation or pressure-induced emission enhancement phenomenon [19, 33, 34]. To explore the reasons for the tiny variation of, we calculated reorganization energy, non-adiabatic electronic coupling and duschinsky rotation effect (DRE) [19]. The reorganization energy is reduced by only 5.30 meV at high pressure. The ignorable change may be contributed to the stable quinone structure of excited states, shown in Fig. S3.Rkk, the diagonal part of the electronic coupling matrix, and DRE also show no obvious change (Figs. S6 and S7 in Supporting information). That is, pressure hardly affects the adiabatic and non-adiabatic process in DPA system. Overall, within a reasonable pressure range, pressure effect can significantly improve the charge mobility of DPA crystals without the loss of fluorescence quantum yield, which is an expected result.
In summary, the charge transport and photophysical properties of high mobility emissive organic semiconductor DPA at different pressures were investigated. Based on first-principles calculation combined with a multi-scale QM/MM method, the hole mobility of DPA would significantly increase with the elevated pressure, from 8.15 cm2V−1 s−1 to 15.72 cm2V−1 s−1. This is mainly due to the significantly enhanced intermolecular interactions, leading to a significant increase in the charge transfer integrals at high pressure. At the same time, the suppressed dihedral rotations and vibrations also benefit to the charge transport properties to a certain extent resulting in a decreased reorganization energy under pressure. For the pressure-induced photophysical properties, there was neither significant piezochromism nor emission enhancement phenomenon founded. The planar morphology and the stable excited state electronic structure make DPA different from the typical AIE and PIEE molecules. Fortunately, the weak exciton coupling effect could not induce fluorescence quenching in the studied pressure, and the quantum yield maintained at a high level ~ 0.47 under pressure. Our work systematically explored the influence of pressure on charge transport and the luminescent properties of organic semiconductor materials. We theoretically explain the pressure response mechanism and propose the external pressure is a simple and efficient way to improve the performance of organic photoelectric materials.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work is supported by National Key R & D Program (No. 2016YFB0401100), the National Natural Science Foundation of China (Nos. 91833306, 51633006).
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi: https://doi.org/10.1016/j.cclet.2020.08.028.
| [1] |
E. Zysman-Colman, Beilstein J. Org. Chem. 14 (2018) 1944-1945. DOI:10.3762/bjoc.14.168 |
| [2] |
C.L. Wang, H.L. Dong, L. Jiang, W.P. Hu, Chem. Soc. Rev. 47 (2018) 422-500. DOI:10.1039/C7CS00490G |
| [3] |
Q. Meng, W.P. Hu, Phys. Chem. Chem. Phys. 14 (2012) 14152-14164. DOI:10.1039/c2cp41664f |
| [4] |
C.Z. Liao, F. Yan, Polym. Rev. 53 (2013) 352-406. DOI:10.1080/15583724.2013.808665 |
| [5] |
Y.L. Guo, G. Yu, Y.Q. Liu, Adv. Mater. 22 (2010) 4427-4447. DOI:10.1002/adma.201000740 |
| [6] |
Z.W. Wang, S.J. Guo, H.W. Li, et al., Adv. Mater. 31 (2019) 10. |
| [7] |
A.S. Li, J. Wang, Y.J. Liu, et al., Phys. Chem. Chem. Phys. 20 (2018) 30297-30303. DOI:10.1039/C8CP06363J |
| [8] |
Y.R. Gu, K. Wang, Y.X. Dai, et al., J. Phys. Chem. Lett. 8 (2017) 4191-4196. DOI:10.1021/acs.jpclett.7b01796 |
| [9] |
Q. Guo, L.J. Wang, F.Q. Bai, et al., RSC Adv. 5 (2015) 18875-18880. DOI:10.1039/C4RA14274H |
| [10] |
Y.G. Zhen, H.L. Dong, L. Jiang, W.P. Hu, Chin. Chem. Lett. 27 (2016) 1330-1338. DOI:10.1016/j.cclet.2016.06.023 |
| [11] |
B. Lu, S.Y. Liu, D.P. Yan, Chin. Chem. Lett. 30 (2019) 1908-1922. DOI:10.1016/j.cclet.2019.09.012 |
| [12] |
Z. Gao, K. Wang, F.M. Liu, et al., Chem. Eur. J. 23 (2017) 773-777. DOI:10.1002/chem.201604923 |
| [13] |
Z.L. Rang, M.I. Nathan, P.P. Ruden, et al., Appl. Phys. Lett. 86 (2005) 3. DOI:10.1063/1.1875761 |
| [14] |
T. Kubo, R. Hausermann, J. Tsurumi, et al., Nat. Commun. 7 (2016) 7. DOI:10.1038/nphys4217 |
| [15] |
S.M. Gali, C. Quarti, Y. Olivier, et al., J. Mater. Chem. C 7 (2019) 4382-4391. DOI:10.1039/C8TC06385K |
| [16] |
K. Sakai, Y. Okada, S. Kitaoka, et al., Phys. Rev. Lett. 110 (2013) 5. |
| [17] |
Z.H. Sun, Q.G. Zang, Q. Luo, et al., Chem. Commun. 55 (2019) 4735-4738. DOI:10.1039/C9CC00978G |
| [18] |
X. Fan, J.L. Sun, F.Z. Wang, et al., Chem. Commun. (2008) 2989-2991. DOI:10.1039/b803539c |
| [19] |
T. Zhang, W. Shi, D. Wang, et al., J. Mater. Chem. C 7 (2019) 1388-1398. DOI:10.1039/C8TC05162C |
| [20] |
N. Li, Y.R. Gu, Y.P. Chen, et al., J. Phys. Chem. C 123 (2019) 6763-6767. DOI:10.1021/acs.jpcc.9b00670 |
| [21] |
Y.R. Gu, N. Li, G.C. Shao, K. Wang, B. Zou, J. Phys. Chem. Lett. 11 (2020) 678-682. DOI:10.1021/acs.jpclett.9b03592 |
| [22] |
X.Y. Deng, H.W. Guo, X. Meng, et al., Chem. Commun. 55 (2019) 4663-4666. DOI:10.1039/C9CC01145E |
| [23] |
H.C. Liu, Y.X. Dai, Y. Gao, et al., Adv. Opt. Mater. 6 (2018) 6. |
| [24] |
H.C. Liu, Y.R. Gu, Y.X. Dai, et al., J. Am. Chem. Soc. 142 (2020) 1153-1158. DOI:10.1021/jacs.9b11080 |
| [25] |
J. Liu, H.T. Zhang, H.L. Dong, et al., Nat. Commun. 6 (2015) 8. |
| [26] |
J.Z. Fan, L. Cai, L.L. Lin, C.K. Wang, J. Phys. Chem. A 120 (2016) 9422-9430. |
| [27] |
Q.Y. Wu, C.M. Deng, Q. Peng, Y.L. Niu, Z.G. Shuai, J. Comput. Chem. 33 (2012) 1862-1869. DOI:10.1002/jcc.23019 |
| [28] |
J.Z. Fan, L.L. Lin, C.K. Wang, Phys. Chem. Chem. Phys. 19 (2017) 30147-30156. DOI:10.1039/C7CP05451C |
| [29] |
M.T. Ruggiero, S. Ciuchi, S. Fratini, G. D'Avino, J. Phys. Chem. C 123 (2019) 15897-15907. DOI:10.1021/acs.jpcc.9b01902 |
| [30] |
H.S. Yuan, K. Wang, K. Yang, B.B. Liu, B. Zou, J. Phys. Chem. Lett. 5 (2014) 2968-2973. DOI:10.1021/jz501371k |
| [31] |
W.Q. Li, Q. Peng, Y.J. Xie, T. Zhang, Z.G. Shuai, Acta Chim. Sin. 74 (2016) 902-909. DOI:10.6023/A16080452 |
| [32] |
Z.Y. Fu, K. Wang, B. Zou, Chin. Chem. Lett. 30 (2019) 1883-1894. DOI:10.1016/j.cclet.2019.08.041 |
| [33] |
X.Y. Zheng, Q. Peng, L.Z. Zhu, et al., Nanoscale 8 (2016) 15173-15180. DOI:10.1039/C6NR03599J |
| [34] |
T. Zhang, H.L. Ma, Y.L. Niu, et al., J. Phys. Chem. C 119 (2015) 5040-5047. DOI:10.1021/acs.jpcc.5b01323 |
 2021, Vol. 32
2021, Vol. 32 

