b Department of Chemistry, Huaibei Normal University, Huaibei 235000, China;
c State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Shanghai 200032, China
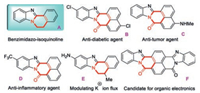
Benzimidazo-isoquinoline fused framework is one of the most important nitrogen-containing heterocyclic units (A, Fig. 1). As a privileged structure, benzimidazo-isoquinoline skeleton is widely found in biologically active natural products, pharmaceutical compounds and material science [1-4], such as anti-diabetic compound (B, Fig. 1) [5, 6], anti-tumor agent (C, Fig. 1) [7, 8], anti-inflammatory compound (D, Fig. 1) [9-11], modulating the potassium ion flux reagent (E, Fig. 1) [12, 13], and candidate for organic electronics (F, Fig. 1) [14]. In addition, benzimidazoisoquinolinone derivatives [15-17], as useful building blocks, are important synthetic intermediates are widely applied in the organic synthesis [18-26]. Owing to their unique properties in biological and material science molecules and their synthetic applications, there is a great need to develop a practical synthetic method to prepare benzimidazoisoquinolinones.
|
Download:
|
| Fig. 1. Selected compounds containing benzimidazoisoquinolines. | |
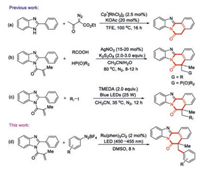
The general procedure for the synthesis of benzimidazoisoquinolinones is limited to multi-step condensation reactions, which suffer from tedious pre-functionalization steps for starting materials, harsh reaction conditions and limited functional group tolerance. Therefore, much effort has been directed towards exploring efficient methods for their synthesis. For example, Song and co-workers developed an elegant Cp*Rh(Ⅲ)-catalyzed [4+2] annulative reaction of 2-arylimidazoles anda-diazoketoesters for the preparation of benzimidazole[2, 1-a]isoquinolines in 2018 (Scheme 1a) [27]. Recently, Yu's group reported a Ag-catalyzed decarboxylative radical cascade cyclization functionalized 2-arylbenzoimidazoles and carboxylic acids into a wide range of benzimidazo[2, 1-a]isoquinoline-6(5H)-ones and a Ag-catalyzed synthesis of phosphoryl-substituted benzimidazo[2, 1-a]isoquinoline-6(5H)-ones from diarylphosphine oxides and 2-arylbenzoimidazoles in one pot (Scheme 1b) [28, 29]. After then, a visible-light promoted radical cyclization to access perfluoroalkyl-substituted benzimidazo[2, 1-a]isoquinolin-6(5H)-ones was described by same group (Scheme 1c) [30]. Despite these achievements, these methods still have some disadvantages, such as the need of expensive metal as catalyst, excess oxidant or additive, and higher reaction temperature, limiting their synthetic applications. So, the development of efficient and convenient route to functionalized benzimidazo[2, 1-a]isoquinolin-6(5H)-ones under mild and environmentally friendly conditions is still desirable.
|
Download:
|
| Scheme 1. Synthesis of benzimidazoisoquinolinone fused frameworks. | |
Visible light is an environmentally friendly and infinitely available energy source that can be used to activate chemical conversions and it induced organic reactions have become a powerful tool in organic synthesis [31-45]. A variety of visible-light induced organic transformationsvia single electron transfer, energy transfer, or a hydrogen atom transfer process have recently received considerable attention [46-48]. In the presence of a photosensitizer, a number of carbon-carbon and carbon-heteroatom bonds formations under visible-light irradiation were reported [49-51]. Herein, we wish to report a simple and efficient visible-light-induced diarylation ofN-methacryloyl-2-arylbenzoimidazoles with aryl diazonium salts using Ru(phen)3Cl2 as a photocatalyst. The reactions generated the corresponding benzimidazoisoquinolinones in good yields through the construction of two C-C bonds in one step under mild reaction conditions (room temperature with the irradiation of blue LEDs in 450–455nm for 8h, Scheme 1d).
Initially, a model reaction of N-methacryloyl-2-phenylbenzoimidazole (1a) with phenyl diazonium salt (2a) was chosen to optimize the reaction conditions. The results are summarized in Table 1. When the model reaction was carried out in the presence of Ru(phen)3Cl2 (3mol%) under air in MeCN with the irradiation of blue light emitting diode (LED, 450–455nm, 3W) for 8h, the desired product 3a was obtained in 52% yield (Table 1, entry 1). As the solvent was changed from MeCN to DMF, the yield of 3a was improved to 61% (entry 2). To our pleasure, an isolated yield of 3a in 66% was achieved when a DMSO was used as the solvent (entry 3). Other solvents such as EtOH and MeOH, were found to be less effective for this reaction (entries 4 and 5). The generally used photocatalysts including eosin Y, rose bengal and acridine red were found to be inferior to Ru(phen)3Cl2 for the reaction, providing 3a in 38%–51% yields (entries 6–9). Ru(bpy)3Cl2 exhibited a comparable activity with Ru(phen)3Cl2, generating 3a in 63% yield (entry 9). When fluorescien or [Acr+-Mes]ClO4 was employed as the photocatalyst instead of Ru(phen)3Cl2 in the model reaction, no desired product 3a was observed (entries 10 and 11). As expected, no reaction occurred in the absence of photocatalyst or visible light (entries 12 and 13). When the reaction was carried out under ambient light, the reaction was much more sluggish and had a dramatically decreased yield (entry 14). Under the irradiation of a LED with 450–455nm or 530–535nm, 43% or 58% yield of 3a was obtained (entries 15 and 16). In addition, the internal temperature of the reaction mixture was measured and found that it is 27±1 ℃.
|
|
Table 1 Optimization of the reaction conditions.a |
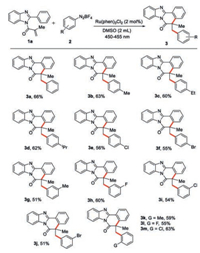
With the optimized reaction conditions in hand, a series of substituted phenyldiazonium salts were examined to establish the substrate scope, and the results are shown in Scheme 2. The reaction of N-methacryloyl-2-phenylbenzoimidazole (1a) with phenyldiazonium salt provided product 3a in 66% yield. Reactions of 1a with aryldiazonium salts with an electron-donating group, such as methyl, ethyl or iso-propyl group, on the para-position of the benzene rings afforded desired products 3b, 3c and 3d in 63%, 60% and 62% yield, respectively. On the other hand, substrates 2 with an electron-withdrawing group, Cl or Br group on the para-position of the benzene rings reacted with 1a to generate the desired products 3e and 3f in 56% and 55% yield, respectively. Moreover, aryldiazonium salts with a halogen group (F, Cl or Br) on the meta-position of benzene rings also delivered the anticipated products 3h–3j in 51%–60% yields. It should be noted that ortho-substituted aryldiazonium salts including 2-methylphenyl-, 2-fluorophenyl-, and 2-chlorophenyldiazonium salts reacted smoothly withN-methacryloyl-2-phenylbenzoimidazole 1a to provide the corresponding products 3k–3m in 55%–63% yields.
|
Download:
|
| Scheme 2. The scope of aryldiazonium salts. Reaction conditions: N-Methacryloyl-2-phenylbenzoimidazole (1a, 0.10 mmol), aryldiazonium salt (2, 0.15 mmol), Ru(phen)3Cl2 (2 mol%), DMSO (2.0 mL) at room temperature (25 ℃) under blue LED (450–455 nm, 3 W) irradiation for 8 h; isolated yield of the product based on 1a. | |
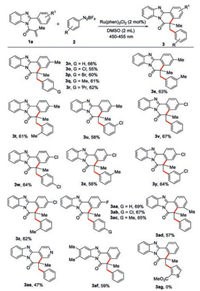
On the other hand, a variety of N-methacryloyl-2-arylbenzoimidazoles were examined to extend the reaction scope of 1, as shown in Scheme 3. N-Methacryloyl-2-(p-methylphenyl)benzoimidazole, N-methacryloyl-2-(p-chlorophenyl)benzoimidazole, and N-methacryloyl-2-(p-fluorophenyl)benzoimidazole reacted with 2a and substituted aryldiazonium salts, including an electron-withdrawing or an electron-donating group, p-chloro, p-bromo, p-methyl, p-iso-propyl, meta-methyl, ortho-methyl, ortho-chloro on the benzene rings, respectively, to afford the corresponding products 3n–3ac in 55%–67% yields, and obvious electronic effect and steric effect are not observed in the formation of products 3n–3ac under the optimal reaction conditions. It should be noted that N-methacryloyl-2-(o-methylphenyl)benzoimidazole and N-methacryloyl-2-pyridinbenzoimidazole are suitable reaction partners with phenyldiazonium salt, providing the anticipated product 3ad in 57% yield, and 3ae in 47% yield, respectively. Reaction of N-methacryloyl-2-phenylbenzimidazole with two methyl groups at the 5- and 6-positions with the diazonium salt 2a to provide the desired product 3af in 59% yield. However, when a thiophene diazonium salt was used as one of the substrates, no desired product 3ag was detected.
|
Download:
|
| Scheme 3. The scope of N-methacryloyl-2-arylbenzoimidazoles. Reaction conditions: N-Methacryloyl-2-arylbenzoimidazole (1, 0.10 mmol), aryldiazonium salt (2, 0.15 mmol), Ru(phen)3Cl2 (2 mol%), DMSO (2.0 mL) at room temperature (25 ℃) under blue LED (450–455 nm, 3 W) irradiation for 8 h; isolated yield of the product based on 1a. | |
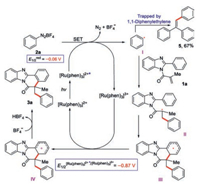
Based on our observation and related literatures [52-58], a plausible pathway for the reaction was depicted withN-methacryloyl-2-phenylbenzoimidazole (1a) and phenyldiazonium salt (2a), as shown in Scheme 4. First, photoexcitation of [Ru(phen)]2+ by visible light generates excited [Ru(phen)]2+* (E1/2Ⅱ*/Ⅲ=−0.87Vvs. SCE for [Ru(phen)]2+*) [59, 60], which is oxidatively quenched by phenyldiazonium salt 2a (E1/2red=−0.06Vvs. SCE) [61]via a single-electron transfer (SET) process to give [Ru(phen)]3+ along with the formation of a phenyl radical Ⅰ. Subsequently, the obtained I undergoes an addition to N-methacryloyl-2-phenylbenzoimidazole 1a to generate the alkyl radical Ⅱ, followed by an intramolecular radical cyclization to give intermediate Ⅲ. A single-electron oxidation of the intermediate Ⅲ by [Ru(phen)]3+ regenerates [Ru(phen)]2+ for next run and forms the intermediate Ⅳ. Ultimately, a BF4- assisted deprotonation yields final product benzimidazo[2, 1-a]iso-quinoline-6(5H)-one 3a.
|
Download:
|
| Scheme 4. The proposed mechanism. | |
In conclusion, we have developed a visible-light-induced photoredox-catalyzed diarylation of N-methacryloyl-2-arylbenzoimidazoles with aryl diazonium salts. The reaction undergoes through a radical casecade process that allows the assembly of benzimidazoisoquinolinones in good yields from two readily accessible building blocks, N-methacryloyl-2-phenylbenzoimidazole and aryldiazonium salts. The method shows considerable synthetic advantages in terms of product diversity and mild reaction conditions.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentWe gratefully acknowledge the National Natural Science Foundation of China (No. 21772062) for financial support.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2020.08.013.
| [1] |
G. Yadav, S. Ganguly, Eur. J. Med. Chem. 97 (2015) 419-443. DOI:10.1016/j.ejmech.2014.11.053 |
| [2] |
M. Goebel, M. Clemenz, B. Staels, et al., ChemMedChem 4 (2009) 445-456. DOI:10.1002/cmdc.200800285 |
| [3] |
Y. Bansal, O. Silakari, Med. Chem. 20 (2012) 6208-6236. |
| [4] |
P. Singla, V. Luxamia, K. Paul, RSC Adv. 4 (2014) 12422-12440. DOI:10.1039/c3ra46304d |
| [5] |
N.I. Kerkvliet, S.K. Kolluri, PCT Int. Appl. 2016, WO 2016069780, EP 3212634, US 20180030048.
|
| [6] |
X. Guo, D. Gu, Z. Wu, et al., Chem. Rev. 115 (2015) 1622-1651. DOI:10.1021/cr500410y |
| [7] |
Y. Chou, R. Chen, Z. Lai, et al., Mol. Cell. Biol. 35 (2015) 2541-2553. DOI:10.1128/MCB.00035-15 |
| [8] |
C.J. Shen, Y. Chou, PCT Int. Appl. 2012, WO 2012112232.
|
| [9] |
J.B. Campbell, E.R. Lavagnino, PCT Int. Appl. US 4413126 A 19831101.
|
| [10] |
J.B. Campbell, E.R. Lavagnino, J.W. Paschal, J. Heterocycl. Chem. 23 (1986) 767-768. DOI:10.1002/jhet.5570230323 |
| [11] |
K. Sun, Y. Si, X. Chen, et al., Asian J. Org. Chem. 8 (2019) 2042-2045. DOI:10.1002/ajoc.201900570 |
| [12] |
X. Wang, A.B. Fulp, PCT Int. Appl. 2005, WO 2005002503, US 20050038067.
|
| [13] |
R. Hili, A.K. Yudin, Nat. Chem. Biol. 2 (2006) 284-287. DOI:10.1038/nchembio0606-284 |
| [14] |
M.J. Taublaender, F. Glöcklhofer, M. Marchetti-Deschmann, et al., Angew. Chem. Int. Ed. 57 (2018) 12270-12274. DOI:10.1002/anie.201801277 |
| [15] |
N.T. Patil, Y. Yamamoto, Chem. Rev. 108 (2008) 3395-3442. DOI:10.1021/cr050041j |
| [16] |
F. Pan, C. Shu, L. Ye, Org. Biomol. Chem. 14 (2016) 9456-9465. DOI:10.1039/C6OB01774F |
| [17] |
W. Xie, Y. Wu, J. Zhang, et al., Eur. J. Med. Chem. 145 (2018) 35-40. DOI:10.1016/j.ejmech.2017.12.038 |
| [18] |
J. Jie, H. Li, S. Wu, et al., RSC Adv. 7 (2017) 20548-20552. DOI:10.1039/C7RA03262E |
| [19] |
A. Obata, A. Sasagawa, K. Yamazaki, et al., Chem. Sci. 10 (2019) 3242-3248. DOI:10.1039/C8SC05063E |
| [20] |
K. Ghosh, Y. Nishii, M. Miura, ACS Catal. 9 (2019) 11455-11460. DOI:10.1021/acscatal.9b04254 |
| [21] |
R. Yang, X. Wu, S. Sun, et al., Synthesis 50 (2018) 3487-3492. DOI:10.1055/s-0037-1610124 |
| [22] |
J. Liu, N. Zhang, Y. Yue, et al., Eur. J. Org. Chem. 34 (2013) 7683-7687. |
| [23] |
J.M. Villar, J. Suarez, J.A. Varela, et al., Org. Lett. 19 (2017) 1702-1705. DOI:10.1021/acs.orglett.7b00478 |
| [24] |
L. Yan, D. Zhao, J. Lan, et al., Org. Biomol. Chem. 11 (2013) 7966-7977. DOI:10.1039/c3ob41760c |
| [25] |
K. Sun, G. Li, Y. Li, et al., Adv. Synth. Catal. 362 (2020) 1947-1954. DOI:10.1002/adsc.202000040 |
| [26] |
M. Zhou, J. Li, C. Tian, et al., J. Org. Chem. 84 (2019) 1015-1024. DOI:10.1021/acs.joc.8b02998 |
| [27] |
S. Mai, Y. Luo, X. Huang, et al., Chem. Commun. 54 (2018) 10240-10243. DOI:10.1039/C8CC05390A |
| [28] |
K. Sun, S. Li, X. Chen, et al., Chem. Commun. 55 (2019) 2861-2864. DOI:10.1039/C8CC10243K |
| [29] |
F. Zeng, K. Sun, X. Chen, et al., Adv. Synth. Catal. 361 (2019) 5176-5181. DOI:10.1002/adsc.201901016 |
| [30] |
K. Sun, Y. Si, X. Chen, et al., Asian J. Org. Chem. 8 (2019) 2042-2045. DOI:10.1002/ajoc.201900570 |
| [31] |
G. Ciamician, Science 36 (1912) 385-394. DOI:10.1126/science.36.926.385 |
| [32] |
G.A. Nicewicz, D.W.C. MacMillan, Science 322 (2008) 77-80. DOI:10.1126/science.1161976 |
| [33] |
K. Zeitler, Angew. Chem. Int. Ed. 48 (2009) 9785-9789. DOI:10.1002/anie.200904056 |
| [34] |
J.M.R. Narayanam, C.R.J. Stephenson, Chem. Soc. Rev. 40 (2011) 102-113. DOI:10.1039/B913880N |
| [35] |
B. Wang, P. Li, T. Miao, et al., Org. Biomol. Chem. 17 (2019) 115-121. DOI:10.1039/C8OB02476F |
| [36] |
T.P. Yoon, M.A. Ischay, J. Du, Nat. Chem. 2 (2010) 527-532. DOI:10.1038/nchem.687 |
| [37] |
C.K. Prier, D.A. Rankic, D.W.C. MacMillan, Chem. Rev. 113 (2013) 5322-5363. DOI:10.1021/cr300503r |
| [38] |
L. Zou, P. Li, B. Wang, et al., Chem. Commun. 55 (2019) 3737-3740. DOI:10.1039/C9CC01014A |
| [39] |
Y. Wu, M. Yan, Z. Gao, et al., Chin. Chem. Lett. 30 (2019) 1383-1386. DOI:10.1016/j.cclet.2019.03.056 |
| [40] |
X. Dai, Y. Li, S. Zhang, et al., Chin. J. Org. Chem. 39 (2019) 1711-1719. DOI:10.6023/cjoc201902022 |
| [41] |
H. Zhang, L. Gu, X. Huang, et al., Chin. Chem. Lett. 27 (2016) 256-260. DOI:10.1016/j.cclet.2015.10.012 |
| [42] |
X. Dai, X. Xu, D. Cheng, et al., Chin. Chem. Lett. 25 (2014) 545-548. DOI:10.1016/j.cclet.2014.01.021 |
| [43] |
Y. Kong, W. Xu, X. Liu, et al., Chin. Chem. Lett. 31 (2020) 3245-3249. DOI:10.1016/j.cclet.2020.05.022 |
| [44] |
W. Ou, R. Zou, M. Han, et al., Chin. Chem. Lett. 31 (2020) 1899-1902. DOI:10.1016/j.cclet.2019.12.017 |
| [45] |
C. Tian, L. Yang, H. Tian, et al., J. Fluor. Chem. 219 (2019) 23-28. DOI:10.1016/j.jfluchem.2018.12.011 |
| [46] |
Y. Chen, L. Lu, D. Yu, et al., Sci. China Chem. 62 (2019) 24-57. DOI:10.1007/s11426-018-9399-2 |
| [47] |
C. Wang, P.H. Dixneuf, J.F. Soulé, Chem. Rev. 118 (2018) 7532-7585. DOI:10.1021/acs.chemrev.8b00077 |
| [48] |
J. Chen, X. Hu, L. Lu, et al., Acc. Chem. Res. 49 (2016) 1911-1923. DOI:10.1021/acs.accounts.6b00254 |
| [49] |
M.D. Kärkäs, J.A. Porco Jr., C.R.J. Stephenson, Chem. Rev. 116 (2016) 9683-9747. DOI:10.1021/acs.chemrev.5b00760 |
| [50] |
K.L. Skubi, T.R. Blum, T.P. Yoon, Chem. Rev. 116 (2016) 10035-10074. DOI:10.1021/acs.chemrev.6b00018 |
| [51] |
N.A. Romero, D.A. Nicewicz, Chem. Rev. 116 (2016) 10075-10166. DOI:10.1021/acs.chemrev.6b00057 |
| [52] |
X. Li, X. Xu, P. Hu, et al., J. Org. Chem. 78 (2013) 7343-7348. DOI:10.1021/jo401069d |
| [53] |
Y. Li, M. Sun, H. Wang, et al., Angew. Chem. Int. Ed. 52 (2013) 3972-3976. DOI:10.1002/anie.201209475 |
| [54] |
T. Xiao, X. Dong, Y. Tang, et al., Adv. Synth. Catal. 354 (2012) 3195-3199. DOI:10.1002/adsc.201200569 |
| [55] |
M. Zhou, R. Song, X. Ouyang, et al., Chem. Sci. 4 (2013) 2690-2694. DOI:10.1039/c3sc50810b |
| [56] |
H. Wang, L. Guo, X. Duan, Chem. Commun. 49 (2013) 10370-10372. DOI:10.1039/C3CC46114A |
| [57] |
Y. Meng, L. Guo, H. Wang, et al., Chem. Commun. 49 (2013) 7540-7542. DOI:10.1039/c3cc44055a |
| [58] |
W. Fu, F. Xu, Y. Fu, et al., J. Org. Chem. 78 (2013) 12202-12206. DOI:10.1021/jo401894b |
| [59] |
R. Young, T. Meyer, D. Whitten, J. Am. Chem. Soc. 98 (1976) 286. DOI:10.1021/ja00417a073 |
| [60] |
D. Nagib, D. MacMillan, Nature 480 (2011) 224-228. DOI:10.1038/nature10647 |
| [61] |
P. Allongue, M. Delamar, B. Desbat, et al., J. Am. Chem. Soc. 119 (1997) 201-207. DOI:10.1021/ja963354s |
 2021, Vol. 32
2021, Vol. 32