b College of Pharmacy and Tianjin Key Laboratory of Molecular Drug Research, Nankai University, Tianjin 300071, China;
c University of Chinese Academy of Sciences, Beijing 100049, China
Amomum tsao-ko Crevost et Lemaire (Zingiberaceae) is a perennial herb mainly distributed in Southwest China and Southeast Asian countries [1, 2]. Its dried fruit as one of the most ancient spices is well-known all over the world [3], which has long been used for treating stomach disorders, throat infections, and liver abscess in China [4, 5]. Previous investigations onA. tsao-ko indicated the presence of diarylheptanoids, flavonoids, monoterpenoids, and essential oils [6-8], many of which showed antimicrobial, antiinflammatory, antioxidant, antiproliferative, and neuroprotective activities [9, 10]. As a continuous search for antidiabetic candidates from natural resources [11], this LCMS and bioassay driven investigation on the dried fruits ofA. tsao-ko afforded two intriguing flavanol-monoterpenoid hybrids, tsaokols A (1) and B (2). Herein, we report their isolation, structural elucidation, plausible biosynthetic pathway, α-glucosidase inhibitory activity, and MS/MS fragmentation.
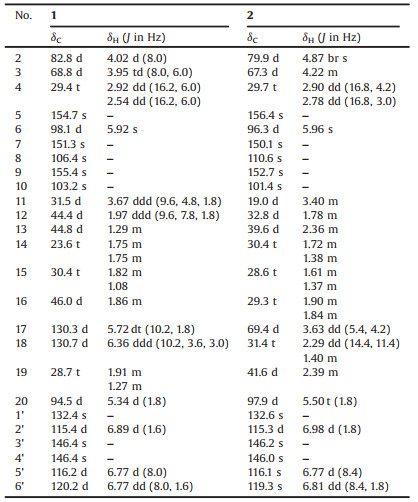
Under the guidance of LCMS analysis and bioassay profiling, the Fr. A-5-2 of A. tsao-ko was detected with two interesting peaks that showed the same molecular formula of C25H26O7 and obvious α-glucosidase inhibitory activity (Fig. S1 in Supporting information). As a result, the two peaks were purified by repeated column chromatography and semi-preparative HPLC on C18 column using MeCN-H2O and MeOH-H2O eluent to yield compounds 1 and 2 (Fig. 1).
|
Download:
|
| Fig. 1. Structures of tsaokols A (1) and B (2). | |
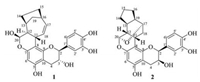
Tsaokol A (1) was obtained as yellowish powder with a molecular formula of C25H26O7 which was deduced by the positive HRESIMS at m/z 439.1752 ([M + H]+, calcd. 439.1751), corresponding to 13 degrees of unsaturation. The IR spectrum exhibited absorption bands for hydroxyl groups (3432 cm−1) and aromatic rings (1621, 1527, and 1447 cm−1). All the 25 carbons were well resolved in the 13C NMR (DEPT) spectrum, involving four methylenes, 13 methines, and eight quaternary carbons. In the 1H NMR data (Table 1), an ABX spin system at δH 6.89 (1H, d, J=1.6Hz, H-2'), 6.77 (1H, d, J=8.0Hz, H-5'), and 6.77 (1H, dd, J=8.0, 1.6Hz, H-6'), an aromatic singlet atδH 5.92 (1H, s, H-6), and two oxygenated methines at δH 4.02 (1H, d, J=8.0Hz, H-2) and 3.95 (1H, td, J=8.0, 6.0Hz, H-3) were indicative for a catechin-like unit, when taking the typical carbons atδC 82.8 (C-2) and 68.8 (C-3) into consideration [12]. This deduction were well confirmed by the correlations of H-2/H-3/H-4 and H-5'/H-6' in the1H-1H COSY spectrum, as well as the HMBC correlations of H-2 with C-9/C-1'/C-2'/C-6', of H-4 with C-5/C-9, and of H-6 with C-8/C-10 (Fig. 2).
|
|
Table 1 1H (600 MHz) and 13C (150 MHz) NMR data of 1 and 2 in CD3OD. |
|
Download:
|
| Fig. 2. Key 1H-1H COSY, HMBC and ROESY correlations of 1 and 2. | |
Apart from the flavanol unit deduced above, ten remnant carbons were assigned as a pair of cis-double bond [δH 5.72 (1H, dt, J=10.2, 1.8Hz, H-17) and 6.36 (1H, ddd, J=10.2, 3.6, 3.0Hz, H-18);δC 130.3 (C-17) and 130.7 (C-18)], five sp3 methines including an oxygenated one (δH 5.34, δC 94.5), and three methylenes. A pure-carbon bicyclo[4.2.1]non-2-ene scaffold was established by detailed inspection of1H-1H COSY data: the correlations of H-11/H-12/H-13/H2-19/H-16/H-17/H-18/H-11, H-13/H2-14/H2-15/H-16, and H-12/H-20. Meanwhile, the key HMBC correlations (Fig. 3) of H-20 with C-11/C-13, H2-19 with C-12/C-14/C-15/C-17, and H-17 with C-11/C-15/C-19 facilitated the above deduction. The flavanol and monoterpenoid parts fused through C-8‒C-11 and C-7‒O‒C-20 bonds were determined by the key HMBC correlations of H-11 with C-7/C-9, H-12/H-18 with C-8, and H-20 with C-7. Thus, the planar structure of compound 1 was elucidated as a flavanol-monoterpenoid hybrid as shown in Fig. 2.
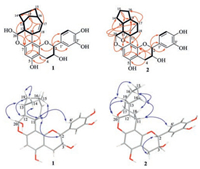
The relative configuration of 1 was characterized by analyzing the coupling constants and ROESY correlations (Fig. 2). The large coupling constants of H-2/H-3 (J=8.0Hz) and of H-11/H-12 (J=9.6Hz) verified theirtrans-axis-orientation. In the ROESY experiment, key cross-peaks from H-11 to H-15a/H-2'(H-6'), from H-12 to H-19b, from H-13 to H-19b and H-19a, from H-16 to H-19b and H-19a, from H-20 to H-13, and from H-2 to H-18 suggested the β-direction of H-11 and OH-20, and α-direction of H-2, H-12, H-13, H-16, H-20, and CH2-19. The same orientation of H-12 and H-20 was consistent with their small coupling constant (JH-12/H-20=1.8Hz). With the anti-form of H-2 and H-3 deduced above, theβ-configuration of H-3 was thus decided.
The absolute configuration of 1 was resolved by ECD calculations (Fig. S2 in Supporting information). The calculated ECD spectrum of 2S, 3R, 11S, 12S, 13S, 16R, 20R-form matched well with the experimental one, by which compound 1 was determined and named as tsaokol A.
Tsaokol B (2) had the same molecular formula of C25H26O7 with 1, which was deduced by its HRESIMS at m/z 439.1743 ([M + H]+, calcd. 439.1751). Detailed analyses of its 1H and 13C NMR data revealed that compound 2 shared a similar flavanol backbone with 1 but distinct in the C10 monoterpenoid part (Table 1). Different from 1, an epi-catechin-like unit was deduced by the small coupling constant of JH-2/H-3 and the chemical shifts of C-2 (δC 79.9) and C-3 (δC 67.3) [12]. Besides the flavanol part, the remaining ten carbons were ascribed as six sp3 methines (including two oxygenated ones) and four sp3 methylenes.
An octahydroindene unit was constructed by the 1H-1H COSY correlations of H-13/H-14/H-15/H-16/H-19/H-13, and H-11/H-12/H-13/H-19/H-17/H-18/H-11, as well as the key HMBC correlations of H-16 with C-13/C-14/C-17, and H-14 with C-12/C-16/C-19. A dioxygenated methine (C-20) directly linking with C-12 was confirmed by H-20 (δH 5.50) that showed 1H-1H COSY correlation with H-12, and HMBC correlations with C-11 and C-13. Similar with 1, the HMBC correlations of H-11 with C-7 and C-9, and of H-20 with C-7 in 2 connected the C10 monoterpenoid and flavanol units by the C-8‒C-11 and C-7‒O‒C-20 linkages. The residual one unsaturation degree required an additional ring in the structure of 2, and thus established the ether bond between C-17 and C-20, which was confirmed by the HMBC correlations from H-20 to C-17 and from H-17 to C-20. In the ROESY spectrum, the correlations of H-11 with H-12, H-14b and H-2'(6'), of H-18a with H-16b, and of H-20 with H-13 and H-19, assigned the β-orientation of H-11 and H-12, the α-orientation of H-2, H-3, H-13, H-19, and the ether linkage between C-17 and C-20. By comparing the calculated and experimental ECD spectra, the absolute configuration of 2 was clarified as 2S, 3S, 11S, 12R, 13S, 17R, 19R, 20S.
Structurally, tsaokols A (1) and B (2) are two unusual flavanol-monoterpenoid hybrids fused with pure-carbon bicyclo[4.2.1]non-2-ene and octahydroindene, respectively. Plausibly biosynthetic pathways for 1 and 2 were proposed in Scheme 1. The flavanol parts (1b and 2b) were widely present in A. tsao-ko, which were condensed with different C10 parts (1a or 2a) by consecutive Michael addition and acetalization to form compounds 1 and 2. Presently, several bicyclononane aldehydes with the skeleton of octahydroindene had been isolated from this species [8], whereas their biosynthetic origin was never discussed. By inspection of the features of 1a and 2a, a key intermediate with three-membered ring was suggested for their transformation. Although the bicyclo[4.2.1]non-2-ene can be well interpreted from the precursor of geranyl pyrophosphate (GPP), monoterpenoids with such a skeleton has not been reported.

|
Download:
|
| Scheme 1. Hypothetical biosynthetic pathways of 1 and 2. | |
Tsaokols A (1) and B (2) were investigated in vitro for their α-glucosidase inhibitory activity, both of which displayed obvious inhibition with IC50 values of 18.8 and 38.6 μmol/L, respectively, much more potent than the positive control, acarbose (IC50 = 213 μmol/L). For better understanding the binding modes of1 and 2 with α-glucosidase, in silico docking study with 3TOP was further performed.
The docking poses of compounds 1 and 2 were shown in Fig. S3 (in Supporting information). The prominent interactions of 1 with enzyme were hydrophobic effects with Phe1559 and Phe1560, hydrogen bonds with Asp1157, Asp1526, Lys1460, Arg1510, Thr1586, and Tyr1251, pi-stacking with Phe1560, and pi-cation with Lys1460. As a comparison, compound2 bonded to enzyme mainly by hydrophobic effects with Trp1369 and Phe1560, hydrogen bonds with Asp1526, Thr1586, Lys1460, and Trp1369, and pi-stacking with Phe1560, Trp1369, and Trp1355.
Tsaokols A (1) and B (2) were further investigated for their MS/MS fragmentation in both positive and negative modes by ESI ion source (Scheme S1 in Supporting information). In the first stage MS, they both showed the [M+H]+ ion at m/z 439 and [M‒H]‒ ion at m/z 437, respectively. When the [M+H]+ ion was selected for MS/MS experiment, compounds 1 and 2 produced quite similar fragmentation ions at m/z 421, 287, and 269, which could be well explained by the neutral loss of H2O, A1, 3 retro-Diels-Alder (RDA) cleavage, and further loss of H2O. Interestingly, their MS/MS fragmentations in negative mode were obviously different. For compound 1, the MS2 ion at m/z 289 was due to the departure of the C10 monoterpenoid part. As a comparison, the characteristic A1, 2 cleavage was occurred in the negative MS2 experiment of 2 yielding the ion at m/z 313. Thus, compounds 1 and 2 could be well differentiated by their respectively diagnostic ions of 437-289 and 437-313 in negative MS mode.
In conclusion, two unusual flavanol-monoterpenoid hybrids, tsaokols A (1) and B (2), with intriguing α-glucosidase inhibitory activity were isolated from A. tsao-ko under the guidance of LCMS and bioassay. Compounds 1 and 2 are a pair of isomers showing quite similar fragmentation pathways in positive MS/MS experiments, whereas they can be fast distinguished by the diagnostic MS2 ions at m/z 289 and 313 in negative mode. Compounds 1 and 2 are two intriguing antidiabetic candidates needing further investigation.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the Yunnan Wanren Project (Nos. YNWR-KJLJ-2019-002 and YNWR-QNBJ-2018-061), the Yunnan Science Fund for Excellent Young Scholars (No. 2019FI017), the Program of Yunling Scholar, the Youth Innovation Promotion Association, CAS (No. Y201756), and the Reserve Talents of Young and Middle-aged Academic and Technical Leaders in Yunnan Province.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2020.08.050.
| [1] |
Y. Yang, R.W. Yan, X.Q. Cai, Z.L. Zheng, G.L. Zou, J. Sci, Food Agric. 88 (2008) 2111-2116. DOI:10.1002/jsfa.3321 |
| [2] |
M.R.T. Rahman, Z.X. Lou, F.H. Yu, P. Wang, H.X. Wang, Saudi J. Bio. Sci. 24 (2017) 324-330. DOI:10.1016/j.sjbs.2015.09.034 |
| [3] |
T.S. Martin, H. Kikuzaki, M. Hisamoto, N. Nakatani, J. Am. Oil Chem. Soc. 77 (2000) 667-673. DOI:10.1007/s11746-000-0107-4 |
| [4] |
L.Q. Yu, N. Shirai, H. Suzuki, et al., J. Nutr. Sci. Vitaminol. 56 (2010) 171-176. DOI:10.3177/jnsv.56.171 |
| [5] |
Y. Yang, Y. Yang, R.W. Yan, G.L. Zou, Bioresource Technol. 101 (2010) 4205-4211. DOI:10.1016/j.biortech.2009.12.131 |
| [6] |
S.S. Hong, J.H. Lee, Y.H. Choi, et al., Tetrahedron Lett. 56 (2015) 6681-6684. DOI:10.1016/j.tetlet.2015.10.045 |
| [7] |
K.Y. Lee, S.H. Kim, S.H. Sung, Y.C. Kim, Planta Med. 74 (2008) 867-869. DOI:10.1055/s-2008-1074552 |
| [8] |
X.Z. Yang, P. Küenzi, I. Plitzko, O. Potterat, M. Hamburger, Planta Med. 75 (2009) 543-546. DOI:10.1055/s-0029-1185320 |
| [9] |
B. Li, H.J. Choi, D.S. Lee, et al., Am. J. Chin. Med. 42 (2014) 1229-1244. DOI:10.1142/S0192415X14500773 |
| [10] |
S.S. Moon, S.C. Cho, J.Y. Lee, Bull. Korean Chem. Soc. 26 (2005) 447-450. DOI:10.5012/bkcs.2005.26.3.447 |
| [11] |
X.F. He, X.K. Zhang, C.A. Geng, et al., Bioorg. Chem. 96 (2020) 103638. DOI:10.1016/j.bioorg.2020.103638 |
| [12] |
C.A. Geng, T.H. Yang, X.Y. Huang, et al., J. Ethnopharmacol. 232 (2019) 39-46. DOI:10.1016/j.jep.2018.12.013 |
 2021, Vol. 32
2021, Vol. 32