b Department of Radiation Oncology, Shanghai Changhai Hospital, Second Military Medical University, Shanghai 200433, China;
c School of Pharmacy, Ningxia Medical University, Yinchuan 750004, China;
d School of Chemical and Environmental Engineering, Shanghai Institute of Technology, Shanghai 201418, China;
e Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China
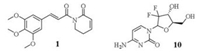
Pancreatic cancer is one of the most aggressive cancers, and chemotherapy remains a main therapeutic strategy for pancreatic cancer treatment in the clinic [1]. Currently, gemcitabine (10, Fig. 1) is used as the standard first-line treatment for patients with pancreatic cancer, exhibiting an improved but unsatisfactory survival rate in recent decades [2]. Many drug combinations with gemcitabine have been evaluated. Among these combinations, gemcitabine plus nab-paclitaxel (Nab-P) exhibited increased survival of pancreatic cancer patients compared with gemcitabine alone [3, 4]. FOLFIRINOX (fluorouracil, oxaliplatin and irinotecan) was approved for the treatment of metastatic pancreatic cancer. The overall survival improved to 11.1 months in the FOLFIRINOX-treated group from 6.8 months in the gemcitabine-treated group [5, 6]. However, this treatment has been used with caution in the clinic due to the high toxicity [7-9]. Only a 2-month improvement in survival (11.1 months vs. 9.2 months) and poor prognosis were observed in those treated with gemcitabine and radiation weekly compared with those who concurrently received a standard dose and schedule of gemcitabine alone [10]. These small improvements hold great promise for the development of effective therapeutic strategies to treat pancreatic cancer, and novel therapeutic agents are also urgently needed.
|
Download:
|
| Fig. 1. The structures of piperlongumine (1) and gemcitabine (10). | |
Piperlongumine (1, Fig. 1), a naturally occurring compound isolated from the root of the plant Piper longum L. [11], exhibits a broad spectrum of biological activities [12]. It has been proposed that reactive oxygen species (ROS) enhancement is the main mechanism of action of compound 1 for killing cancer cells [13]. In our previous report, a preliminary structure-activity relationship (SAR) study showed that the two olefins were critical for the cytotoxicity [14], and introduction of a chloride on the lactam had favorable effects on the activity [15]. In this study, structure optimization was focused on the lactam, conducting ring expansion with one additional carbon, and the substituents on the benzene ring. Representative compounds were selected for pancreatic cancer cell growth inhibition. These compounds were used as effective radiosensitizers of pancreatic cancer cells in vitro and in vivo, and the underlying mechanisms were elucidated. The synthetic routes of the target compounds were performed in Scheme S1 (Supporting information).
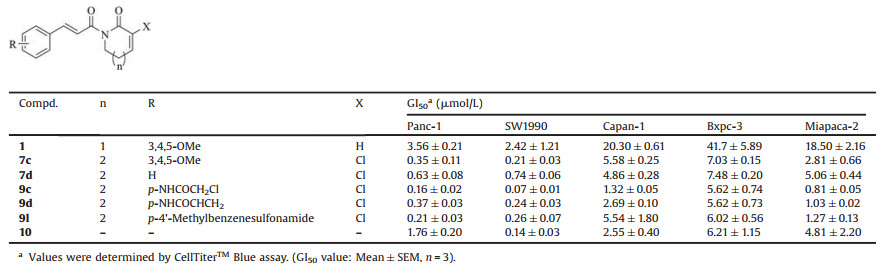
Based on our previous SAR results, modification of the double bond of the lactam using chlorine was beneficial to the activity of the compound, probably due to enhancement of the reactivity of the Michael acceptor [15]. As shown in Table S1 (Supporting information), we tested the concentration that causes 50% growth inhibition (GI50) of piperlongumine derivatives against Panc-1 cells, and gemcitabine was selected as control. Based on the results, an SAR of piperlongumine derivatives has been summarized as Scheme S2 (Supporting information). Then, the pancreatic cancer cell growth inhibition profile was elucidated. Five derivatives (7c, 7d, 9c, 9d and 9l) were selected by the high cytotoxicity toward Panc-1 cells and their chemical structures. Compound 7c with trimethoxyl group and 7d without substitution were selected from 7a-7g. Compound 9c were selected due to its better potency (GI50=0.16±0.02 μmol/L) than that of compound 9b (GI50=0.52±0.17 μmol/L), which had a same substitution on a different position. Similarly, 9d and 9l were selected for the acrylamide group and methylphenyl group. As shown in Table 1, four other pancreatic cell lines (SW1990, Capan-1, Bxpc-3 and Miapaca-2) were used in this assay. These compounds exhibited excellent growth inhibitory profiles, similar to that of 10. Panc-1 and SW1990 were the most sensitive cell lines among the five pancreatic cancer cell lines. The selected compounds exhibited higher potency than compounds 1 and 10 against these two cell lines. Compound 9c had the best potency toward SW1990 cells, with a GI50 value of 70 nmol/L, which was more than 30-fold higher than that of compound 1 and 2-fold higher than that of compound 10. The selected compounds exhibited relatively low sensitivity in the other three cell lines. Compound 1 exhibited GI50 values of only 18.5–41.7 μmol/L. The five compounds exhibited significantly improved potency by 4~20-fold. In particular, compound 9c had a GI50 of 1.32±0.05 μmol/L against Capan-1 cells, which was 15-fold higher than that of compound 1 and 2-fold higher than that of compound 10. Against Miapaca-2 cells, compound 9c exhibited a GI50 in the nanomolar range (0.81±0.05 μmol/L), and compound 9d had a GI50 of 1.03±0.02 μmol/L, which was 18~22-fold higher than that of compound 1 and much higher than that of compound 10 (GI50=4.81±2.20 μmol/L).
|
|
Table 1 The pancreatic cancer cell growth inhibitory profiles of the five selected piperlongumine derivatives. |
In order to determine the selectivity of piperlongumine derivatives toward normal cells, we evaluated the cytotoxicity of 9c and 9d against three human normal cell lines, which were hTERT-HPNE (pancreatic epithelium cells), HKC (renal tubular epithelium cells) and HIEC (Intestinal epithelium cells). Panc-1 and SW1990 cells, which are sensitive to piperlongumine treatment, were selected (Table S2 in Supporting information). Both compounds showed significant selectivity index to cancer cell lines. The GI50 values of 9c were 0.16±0.02 μmol/L and 0.07±0.01 μmol/L against Panc-1 and SW1990, while much lower cytotoxicity was observed in normal cells [hTERT-HPNE cells (1.47±0.24 μmol/L, 9-fold, 21-fold), HKC cells (1.11±0.05 μmol/L, 7-fold, 16-fold) and HIEC cells (7.90±0.24 μmol/L, 49-fold, 113-fold)]. The GI50 values of 9d were 0.37±0.03 μmol/L and 0.24±0.03 μmol/L against Panc-1 and SW1990, and 2.26±0.21 μmol/L against hTERT-HPNE cells (6-fold, 9-fold), 4.16±0.06 μmol/L against HKC cells (11-fold, 17-fold) and 12.01±0.18 μmol/L against HIEC cells (32-fold, 50-fold). However, the parent piperlongumine (1) showed much lower selectivity index (1~9-fold) toward three normal cells. Collectively, the new derivatives selectively inhibit pancreatic cancer cell growth.
We then determined the potential radiosensitizing effect of piperlongumine derivatives in pancreatic cancer cells (Panc-1 and SW1990 cells) using a long-term clonogenic assay. The GI20 were used to obtain suitable therapeutic windows for radiosensitivity evaluation [16, 17]. The GI20 values of the compounds 1, 7c, 7d, 9c, 9d and 10 against Panc-1 were 409, 27, 206, 100, 130, and 47 nmol/L, respectively, and against SW1990, the values were 650, 40, 40, 20, 20, and 20 nmol/L, respectively (Fig. S1 in Supporting information). Then, this dosing regimen was used to test the radiosensitization effects of these compounds. Cells were pretreated with the compounds for 24 h, followed by irradiation at different doses up to 8Gy. Compounds were then washed out after 48 h of irradiation. Then, the cells were cultured in compound-free medium for an additional 7–9 days, allowing colony formation (Fig. S2 in Supporting information). Under these conditions, compounds 9c and 9d effectively sensitized both pancreatic cancer cell lines to radiation with sensitivity enhancement ratios (SERs) of 1.69, 1.45 (Panc-1) and 1.13, 1.30 (SW1990), respectively. Compound 7d exhibited an SER of 1.22 toward Panc-1 cells and only 0.98 toward SW1990 cells. Compounds 1 and 7c exhibited no apparent sensitization with SERs of ~1.0 toward the two cell lines. The combination of compound 10 (GI20: 20 nmol/L) and radiation therapy resulted in a distinct SER of 1.49 in Panc-1 cells, while in SW1990 cells, the SER value (1.09) was not high. Thus, we concluded that compounds 9c and 9d are potent radiosensitizers in pancreatic cancer cells.
The Panc-1 and SW1990 xenograft models were initially tried, with only Panc-1 model growing well. Considering our scope of the piperlongumine-derivative-mediated radiosensitization, we evaluated the compound only in the Panc-1 xenograft model. Compounds 1 (5 mg/kg, i.p./day, 14 days) and 10 (25 mg/kg, i.p., twice a week for 2 weeks) were selected as controls. Panc-1 cells were inoculated subcutaneously into both flanks of nude mice to compare the activities using the same mouse [17]. Radiation was delivered directly to the tumor, with the rest of the animal shielded by using an in-house lead brick. As shown in Fig. 2 and Fig. S3 (Supporting information), administration of compound 1, 9c or 9d alone, at a dose of 5.0 mg/kg, i.p./day for 14 days, led to tumor growth inhibitions (TGIs) of 31%, 50% and 34%, respectively, which were significantly lower than that observed with compound 10 (62%). In addition, no obvious tumor inhibitory activity (only 14%) was observed using radiation as a single treatment at a dose of 6Gy per day for two weeks. In response to treatment with the combination of piperlongumine derivatives and radiation, tumor growth was significantly inhibited compared with the effect of either treatment alone at day 14. The inhibition rate of compound 1 increased from 31% to 44%. The inhibition rate of compound 10 increased from 62% to 73%. The inhibition rates of compounds 9c and 9d increased from 50% to 73% and 34%–59%, respectively. Importantly, the combination treatment was well tolerated by the animals, with a loss of body weight lower than that observed with Food and Drug Administration (FDA) approved drug 10. The tumors were then collected to confirm the inhibitory effects. Our results showed that compounds 9c and 9d effectively sensitized Panc-1 tumor growth to radiotherapy.

|
Download:
|
| Fig. 2. Piperlongumine-derivative-mediated radiosensitization in Panc-1 xenograft tumor model. (A–B) Tumor volume in each group of nude mice. (C) Body weight in each group of nude mice. (D) Tumor inhibition rate statistics. *P < 0.05, **P < 0.01. Control is the group treated with normal saline (i.p./d, 14 d). | |
We next explored the mechanisms of action of piperlongumine derivatives at the cellular level. ROS accumulation in pancreatic cancer cells treated with piperlongumine derivatives was evaluated using the redox-sensitive fluorescent probe 2′, 7′-dichlorofluorescein diacetate (DCFH-DA, Fig. S4 in Supporting information). Consistent with the observed in vitro and in vivo efficacy, compounds 9c and 9d exhibited the best ROS induction compared with the other derivatives in both Panc-1 and SW1990 cells. The Keap1-Nrf2 pathway is of great importance for the clearance of cellular ROS and maintenance of the stability of the intracellular environment [18]. As shown in Fig. 3A, Nrf2 levels in compounds 9c and 9d treated Panc-1 and SW1990 cells were significantly upregulated in a dose-response manner (10 nmol/L and 100 nmol/L) at 12 h. Accordingly, Keap1, a suppressor of Nrf2, was significantly downregulated with high doses of compounds 9c and 9d at 12 h. The regulations were much better than the parent piperlongumine (1) in the same concentrations. For NQO-1 and HO-1, two downstream antioxidant enzymes, no significant changes have been demonstrated for compound 1 treated cells. Compounds 9c and 9d could markedly increase the expressions of NQO-1 in a dose-dependent manner in Panc-1 and SW1990 cells at 12 h. These two compounds could also increase the expressions of HO-1 in Panc-1 and SW1990 cells at 12 h. In addition, 9c and 9d could induce up-regulation of γ-H2AX expression and no changes in total H2AX in two cell lines, indicating DNA damage occurred.

|
Download:
|
| Fig. 3. Piperlongumine derivatives affect protein expression in pancreatic cancer cells. (A) Effects of different concentrations of piperlongumine derivatives on the expression of proteins in Panc-1 and SW1990 cells. (B) Effect of piperlongumine derivatives combined with radiotherapy on intracellular protein expression in Panc-1 and SW1990 cells. (C) Expression of the Nrf2 protein in Panc-1 nucleus and cytoplasm. (D) Immunofluorescence analysis of the effects of piperlongumine derivatives combined with radiotherapy on γ-H2AX protein expression in Panc-1 and SW1990 cells (magnification: × 400). (E) Chemical structures of probes 19a and 19b. (F) Streptavidin pull down of 19a and 19b.Control is the group treated with DMSO group (0.1%). | |
To verify whether the Nrf2 protein was involved in the transcription of downstream proteins and translocated into the cell nucleus from the cytoplasm, we examined the levels of the Nrf2 protein in the nucleus and cytoplasm using piperlongumine derivatives (100 nmol/L) at different time points. As demonstrated in Fig. 3C, the expression of Nrf2 was significantly translocated into the nucleus, peaked at 6 h, and then, the trend was reversed, as detected at 12 h and 24 h. Nrf2 expression decreased steadily throughout the process in the cytoplasm. We finally tested the effect of combination therapy on protein expression. As shown in Fig. 3B, Nrf2 and its downstream protein HO-1 and NQO-1 were obviously increased by compounds 9c and 9d with radiation (6Gy) in a time-dependent manner. Consistent with the SER value, compound 1 did not affect Nrf2 and HO-1 expression after irradiation (6Gy). Given that radiation is closely associated with DNA damage [19], the expression of γ-H2AX was also significantly enhanced by compounds 1, 9c and 9d. These results were also confirmed by the immunofluorescence assay (Fig. 3D, Fig. S5 in Supporting information). The intracellular protein fluorescence intensity was significantly increased by the combination of the compounds and radiation. In particular, compounds 9c and 9d exhibited a greater than 2-fold increase in fluorescence intensity in combination with radiotherapy and better effects than compound 1. The above results indicate that γ-H2AX plays a predominant role in combination radiotherapy.
The piperlongumine is considered to be a pan-assay-interference-compounds (PAINS) compound due to the Michael receptor [20, 21]. In order to address the concern, we first screened our analogues (9c and 9d) through the PAINS compound databases and they both passed the filter (Fig. S6 in Supporting information). Second, PAINS compounds typically had a confusing SAR [20, 21]. However, the newly obtained piperlongumine derivatives have a clear SAR (Scheme S2 in Supporting information). Third, based on the mechanism study, the Keap1-Nrf2 pathway is potentially involved. In order to further elucidate the potential interaction of the compounds with Keap1, a pull-down assay [14, 22, 23] were performed. We synthesized two biotinylated piperlongumines (19a and 19b, Fig. 3E, Scheme S3 in Supporting information) by using click reaction [24] and they showed moderate cytotoxic effect on Panc-1 cells (Fig. S7 in Supporting information). As shown in Fig. 3F, Keap1 was dose-dependently pulled down by the probes in Panc-1 cell lysates, which could be completely competed by the parent 9c and 9d. Thus, our data indicate that the piperlongumines bind to Keap1 in Panc-1 cells instead of a promiscuous one.
To further determine the nature of piperlongumine-mediated radiosensitization, we carried out cell cycle profiling of two cell lines treated with compounds 1, 9c and 9d; radiation; or compounds in combination with radiation (Fig. S8 in Supporting information). The apoptotic rate improved obviously as radiation added, and the effects were improved by the combination of 1, 9c or 9d in both cell lines. The G2/M phase block is a marker of DNA damage [25]. Consistent with the expression of the γ-H2AX protein, the G2/M phase ratio of the cells increased significantly after radiation, suggesting increased DNA damage. Compounds 9c and 9d enhanced the radiation-induced G2/M arrest in Panc-1 and SW1990 (P < 0.05). Taken together, these results show that the piperlongumine derivatives combined with radiotherapy could enhance cellular DNA damage and have a certain sensitizing effect, leading to apoptosis.
In summary, our study revealed the radiosensitizing activity of piperlongumine derivatives in pancreatic cancer and elucidated the mechanisms of action of these compounds in the induction of ROS expression and regulation of the Keap1-Nrf2 protective pathway by targeting Keap1 protein in parallel with enhancement of radiation-induced DNA damage, G2/M-phase cell cycle arrest, and apoptotic death. In a pancreatic bi-flank xenograft tumor model, 9c and 9d significantly inhibited tumor growth with high TGI under radiation. Therefore, our proof-of-concept study provides the first preclinical evidence for the future development of piperlongumine derivatives as novel radiosensitizing agents against pancreatic cancer and, possibly, other types of human cancers.
Declaration of competing interestThe authors declare no conflicts of interest.
AcknowledgmentsThis work was supported by grants from the Shanghai Municipal Commission of Health and Family Planning (No. 2017YQ052), the Young Elite Scientists Sponsorship Program by the China Association for Science and Technology (No. 2017QNRC061), the National Natural Science Foundation of China (Nos. 81673352, 81872453), the Bio-Pharmaceutical Project of Science and Technology of Shanghai (No. 15431901700), the Natural Science Foundation of Shanghai (No. 18ZR1438700) and the Key Research and Development Program of Ningxia (Nos. 2018BFH02001 and 2019BFG02017).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.08.049.
| [1] |
X. Zhang, Q. Liu, Q. Liao, Y. Zhao, J. Cancer 11 (2020) 2749-2758. DOI:10.7150/jca.37445 |
| [2] |
J. Dai, Y. Zhang, H. Li, et al., Chin. Chem. Lett. 28 (2017) 531-536. DOI:10.1016/j.cclet.2016.11.008 |
| [3] |
P.A. Philip, J. Lacy, F. Portales, et al., Lancet Gastroenterol. Hepatol. 5 (2020) 285-294. DOI:10.1016/S2468-1253(19)30327-9 |
| [4] |
A.S. Azmi, H.Y. Khan, I. Muqbil, et al., Clin. Cancer Res. 26 (2020) 1338-1348. DOI:10.1158/1078-0432.CCR-19-1728 |
| [5] |
U.M. Vogl, H. Andalibi, A. Klaus, et al., BMC Cancer 19 (2019) 28-36. DOI:10.1186/s12885-018-5240-6 |
| [6] |
I.R. Cho, H. Kang, J.H. Jo, et al., World J. Gastrointest. Oncol. 12 (2020) 182-194. |
| [7] |
T. Kamisawa, L.D. Wood, T. Itoi, K. Takaori, Lancet 388 (2016) 73-85. DOI:10.1016/S0140-6736(16)00141-0 |
| [8] |
M. Suker, B.R. Beumer, E. Sadot, et al., Lancet Oncol. 17 (2016) 801-810. DOI:10.1016/S1470-2045(16)00172-8 |
| [9] |
T. Conroy, J.B. Bachet, A. Ayav, et al., Eur. J. Cancer 57 (2016) 10-22. DOI:10.1016/j.ejca.2015.12.026 |
| [10] |
N.H. Tran, V. Sahai, K.A. Griffith, et al., Int. J. Radiat. Oncol. Biol. Phys. 106 (2020) 124-133. DOI:10.1016/j.ijrobp.2019.08.057 |
| [11] |
A. Chatterjee, C.P. Dutta, Sci. Cult. 29 (1963) 568-570. |
| [12] |
S. Prasad, A.K. Tyagi, Curr. Pharm. Des. 22 (2016) 4151-4159. DOI:10.2174/1381612822666160601103027 |
| [13] |
J. Zhou, Z. Huang, X. Ni, C. Lv, Toxicol. In Vitro 65 (2020) 104775. DOI:10.1016/j.tiv.2020.104775 |
| [14] |
C. Zhuang, W. Zhang, C. Sheng, et al., Chem. Rev. 117 (2017) 7762-7810. DOI:10.1021/acs.chemrev.7b00020 |
| [15] |
Y. Wu, X. Min, C. Zhuang, et al., Eur. J. Med. Chem. 82 (2014) 545-551. DOI:10.1016/j.ejmech.2014.05.070 |
| [16] |
Z. Wang, S.T. Lai, N.Y. Ma, et al., Cancer Lett. 369 (2015) 192-201. DOI:10.1016/j.canlet.2015.08.015 |
| [17] |
D. Wei, H. Li, J. Yu, et al., Cancer Res. 72 (2012) 282-293. DOI:10.1158/0008-5472.CAN-11-2866 |
| [18] |
D. Schmoll, C.K. Engel, H. Glombik, Discov Drug, Today 24 (2017) 11-17. DOI:10.1016/j.ddtec.2017.10.001 |
| [19] |
M.D. Xu, S.L. Liu, B.B. Zheng, et al., Pancreatology 3903 (2018) 30645-30648. |
| [20] |
J.B. Baell, G.A. Holloway, J. Med. Chem. 53 (2010) 2719-2740. DOI:10.1021/jm901137j |
| [21] |
S.J. Capuzzi, E.N. Muratov, A. Tropsha, J. Chem. Inf. Model. 57 (2017) 417-427. DOI:10.1021/acs.jcim.6b00465 |
| [22] |
B. Zhou, X. Yu, C. Zhuang, et al., ChemMedChem 11 (2016) 1436-1445. DOI:10.1002/cmdc.201600150 |
| [23] |
J. Lin, X.D. Li, Chin. Chem. Lett. 29 (2018) 1051-1057. DOI:10.1016/j.cclet.2018.05.017 |
| [24] |
H. Chen, Y. Jiang, Y. Zhang, et al., Chin. Chem. Lett. 28 (2017) 913-918. DOI:10.1016/j.cclet.2016.11.027 |
| [25] |
F. Salehi, H. Behboudi, G. Kavoosi, S.K. Ardestani, Sci. Rep. 8 (2018) 13902. DOI:10.1038/s41598-018-32308-2 |
 2021, Vol. 32
2021, Vol. 32