b State Key Laboratory of Elemento-Organic Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China
Selective separation of carbon dioxide (CO2) and acetylene (C2H2) from methane (CH4) are of significant challenges in natural gas purification and industrial acetylene production [1, 2]. However, the conventional porous materials such as zeolites and porous carbons, polymer-based membranes and cryogenic distillation technology cannot meet the requirements for selective separation of the impurities with the similar molecular kinetic diameter (CO2: 3.3Å, C2H2: 3.3Å, CH4: 3.758Å), boiling point (CO2: 216.55 K, C2H2: 188.40 K, CH4: 111.66 K) and polarizability (CO2: 29.11 × 1025 cm−3, C2H2: 33.3-39.3 × 1025 cm−3, CH4: 25.93 × 1025 cm−3) [3]. Moreover, the low efficiency and high energy consumption during the separation process also made them less compatible [4]. Therefore, the development of new materials for CO2/CH4 and C2H2/CH4 separation are of great needs.
Metal-organic frameworks (MOFs), as crystalline porous materials, have been widely studied in the areas of gas storage and separation [5-9], luminescent sensing [10-12], heterogeneous catalysis and electrochemistry [13-15]. Benefiting from their unique structures, MOFs are regarded as ideal materials for effectively binding and selective separation of CO2/CH4 and C2H2/CH4. Moreover, the rich active heteroatoms sites and open metal sites (OMSs) can enhance the preferential binding of specific guest molecules. For instance, He et al. reported a rare Pb9-cluster MOF based on a flexible cyclotriphosphazene-functionalized hexacarboxylate ligand for gas separation [16]. Owing to the rich active heteroatoms sites and OMSs on the inner pore surfaces of the MOF, the adsorption selectivities of the MOF for the equimolar CO2/CH4 and C2H2/CH4 at 298 K and 100 kPa calculated by the predicted ideal adsorbed solution theory (IAST) are 5.8 and 21.0, respectively. However, it should be noted that the rational predesign and construction of MOF materials containing specific microporous and multiple functional active sites (OMS, -NH2, -F, -OH and etc.) are still challenging [17-19]. On one hand, the various coordination modes of both center metal ions and organic ligands make it complicated to predict the final structures of the self-assembly MOFs; On the other hand, the obtained MOF materials may not be applicable to the gas separation on account of the unsuitable aperture of MOF channels and the lack of functional active sites for binding with gas molecules.
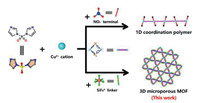
In order to tackle the intractable problem, we promoted a cross-linking strategy for the construction of porous MOF materials with multifunctional active sites for effectively selective separation of gas mixtures. A previously reported coordination polymer (CP) [Cu(sdi)2(NO3)2]·2CO(CH3)2 was a representative example for illustrating the strategy [20]. In the titled CP, the one-dimensional (1D) infinite coordinate chains are held together to form three-dimensional (3D) supramolecular structure via hydrogen-bonding and Van der Waals interactions. It is worth noting that these 1D chains possess large amounts of terminal coordinated NO3− moieties with the hexa-coordinated metal Cu(Ⅱ) ions. Inspired by this, if the initial metal salt was changed from Cu(NO3)2·3H2O to CuSiF6·nH2O, then the SiF62- moieties were expected to instead of the terminal NO3−, and ultimately these separated 1D chains would be cross-linked by the SiF62- moieties to build a novel microporous 3D network (Scheme 1). In addition, the SiF62- moieties can also act as functional active sites to facilitate the selective separation of gas molecules, which is proved by previously reported works [21, 22].
|
Download:
|
| Scheme1. Schematic for the cross-linking construction of the 3D microporous MOF. | |
With this in mind, we successfully constructed a 3D microporous MOF material [Cu(SiF6)(sdi)2]·solvents (1). Compound 1 possessed one-dimensional (1D) narrow channels along the c direction, and large amounts of O and F donor sites originated from -SO2- and SiF62− on the inner walls of the channels. These dense functional active sites can provide strong binding affinities to specific guest molecules, and thus effectively improve the gas separation performance. Gas adsorption experiments proved that 1a (activated 1) has high adsorption capacity and selectivity for CO2/CH4 and C2H2/CH4. In addition, the MOF exhibits high stability not only in common organic solvents but also in deionized water, which is beneficial to its practical and industrial applications.
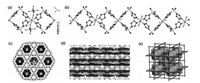
Single-crystal X-ray diffraction measurement reveals that compound 1 crystallized in the trigonal system with the R 3 c space group. The asymmetric unit of1 contains one crystallographically independent sdi ligand, one half of Cu(Ⅱ) cation and SiF62− anion. As shown in Fig. 1a, the Cu(Ⅱ) centre is hexa-coordinated with four nitrogen atoms originated from four different sdi ligands and two fluorine atoms provided by two SiF62− anions. The Cu-N and Cu-F bond distances are in the normal range of 1.988(2)-2.4472(19) Å, which are comparable to the other similar MOFs reported in the previously published papers [23-25]. The selected bond length and angles are listed in Table S2 (Supporting information). Each sdi ligand is coordinated with two Cu(Ⅱ) ions and connected with another one sdi ligand to generate a [Cu2sdi2] unit, and the adjacent units by sharing Cu(Ⅱ) ions to form an infinite 1D chain (Fig. 1b). Finally, the 1D chains are further cross-linked by SiF62− groups to form a 3D honeycomb-like structure with 1D narrow channels along the c axis (Figs. 1c and d). The pore limiting diameter of 1 is around 3.4 Å viewed from c axis and the potentially free volume (without the solvent molecules) estimated by PLATON program is 25.9%. In order to better understand the complicated structure of 1, ToposPro software is applied to simplify the specific structure [26]. Based on the simplification, compound1 can be regarded as a novel 4-connected unimodal nbo PLATON program is 25.9 of {64.82} (Fig. 1e).
|
Download:
|
| Fig. 1. (a) The coordination environment of the centre Cu(Ⅱ) ion. (b) The infinite 1D Cu(Ⅱ)-sdi chain (SiF62− groups are omitted for clarity). (c, d) The 3D structure of 1 along c and b axes (the isosurfaces represent the voids inside the pores). (e) The tiling image of the simplified framework of 1. Color codes: Cu (sky blue), S (yellow), Si (orange), F (green), O (red), N (mazarine), C (gray). Hydrogen atoms are omitted for clarity. | |
The purity of 1 was proved by PXRD. As shown in Fig. S2 (Supporting information), the observed PXRD pattern matched well to the simulated one, indicating the bulk sample of 1 possessed good phase purity. TGA curve reveals that 1 has moderate thermal stability and can keep its structure until 230 ℃. In addition, 1 can maintain its pristine crystal structure not only in common organic solvents (MeOH, EtOH, CH3COCH3, DMF, DMAC, DMSO, CH3CN, CH2Cl2, CHCl3), but also in deionized water for three days, which enhances its potential for practical application (Fig. S3 in Supporting information).
Based on the good stability of 1, N2 adsorption experiment was performed at 77 K to evaluate the porosity of1a. As illustrated in Fig. S4 (Supporting information), negligible N2 was adsorbed. The sample was collected after the N2 sorption measurement for further inspection. PXRD testing confirmed that the framework of 1a did not collapse (Fig. S2), so the low N2 uptake of 1a can be attributed to the repulsion of the framework to N2 and the small aperture size hindered the N2 molecules into the channels of 1a under this circumstance. Subsequently, we changed the probe molecule to CO2 because of its small kinetic diameter (3.30Å) compared to N2 (3.64 ~ 3.80Å). As expected, a high uptake amount of CO2 with type I isotherm was observed at 195 K (saturated capacity: 101.2 cm3/g, Fig. S4). The experimental pore volume of 1a is 0.1512 cm3/g. The Brunauer-Emmett-Teller (BET) surface area and Langmuir surface area of 1a were calculated to be 358 m2/g and 442 m2/g based on the CO2 adsorption experiment, respectively [27, 28].
The microporous structure with relatively high BET surface area, multiple functional active sites and high stability prompted us to investigate the gas sorption and separation properties of 1a. CO2, C2H2 and CH4 were selected as the targeted molecules on account of their high-purity industrial demand and scientific research. The single-component adsorption experiments of 1a toward CO2, C2H2 and CH4 were measured at 273 K and 298 K, respectively. As illustrated inFig. 2a, 1a has relatively high CO2 and C2H2 adsorption capacity (29.2 cm3/g for CO2 and 34.2 cm3/g for C2H2) at 298 K and 1 atm. When the temperature is reduced to 273 K, the trend is maintained and the corresponding gas uptakes increased to 45.1 and 48.7 cm3/g, respectively (Fig. 2b). Compared to CO2 and C2H2, only limited amounts of CH4 are adsorbed (6.8 cm3/g at 298 K and 11.7 cm3/g at 273 K). The distinctly different gas adsorption behaviors indicated that1a can act as a promising material for the selective separation of CO2/CH4 and C2H2/CH4.

|
Download:
|
| Fig. 2. Single-component adsorption isotherms of C2H2 (black), CO2 (red), CH4 (blue) of 1a at (a) 298 K and (b) 273 K. | |
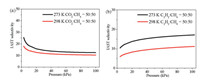
The adsorption selectivities of 1a towards binary gas mixtures CO2/CH4 (50/50, v/v) and C2H2/CH4 (50/50, v/v) were evaluated by the IAST. The Langmuir-Freundlich equation fits extremely well with the single-component isotherms of CO2, C2H2 and CH4 at 273 and 298 K (Figs. S5-S7 in Supporting information). As shown inFig. 3, the IAST adsorption selectivities are 10.4 for CO2/CH4 and 11.2 for C2H2/CH4 at 298 K. As the temperature lowered to 273 K, the selectivities increased to 12.5 and 17.1, respectively. The IAST selectivities of1a towards CO2/CH4 (50/50, v/v) and C2H2/CH4 (50/50, v/v) under 298 K are superior to some previously reported MOFs (Table S3 in Supporting information). These results further proved that1a is capable for selective separation of CO2/CH4 and C2H2/CH4 mixtures.
|
Download:
|
| Fig. 3. The IAST adsorption selectivities of CO2/CH4 and C2H2/CH4 of 1a at 273 K (black) and 298 K (red). | |
To elucidate the reason that of compound 1a exhibits comparatively high adsorption amount and selectivity for CO2 and C2H2, the Qst for 1a toward the different gas molecules were calculated by the Clausius-Clapeyron equation using isotherm fits to the Virial equation based on the single component gas adsorption isotherms measured at 273 K and 298 K (Figs. S8-S10 in Supporting information). The Qst values at near zero coverage are found to be 31.9, 26.8 and 16.3 kJ/mol for CO2, C2H2 and CH4, respectively (Fig. S11 in Supporting information). Obviously, the Qst values of CO2 and C2H2 are much higher than that of CH4, which confirmed the strong affinities between CO2, C2H2 molecules and the framework of 1a, and thus resulting the highly selective separation of CO2/CH4 and C2H2/CH4. The Qst curves for CO2 and C2H2 showed two distinctly different trends, with the increase of gas adsorption, the Qst value for CO2 decreased, while that for C2H2 increased. These phenomena could be ascribed to the continuously variational intermolecular interactions along with the gas adsorption process [29]. Moreover, the Qst values of both CO2 and C2H2 are comparable to or even surpassing to some well-known MOFs decorated with functional group sites (-NH2, -F, -OH, etc.) and/or OMSs (Table S4 in Supporting information).
Therefore, on the basis of the above analysis, the following aspects can be used as explanation of the preferable adsorption phenomena of 1a: (a) The small apertures and the repulsion of framework hindered the CH4 molecules into the pore channels of 1a [30]; (b) The O and F donor sites originated from the functional active sites (-SO2- and SiF62−) on the inner walls of the channels can provided strong binding affinities via hydrogen-bonding and/or Van der Waals interactions with the CO2 and C2H2 molecules [31, 32]; (c) CO2 and C2H2 are more easily to be condensed than CH4 for their relatively higher critical temperature (CO2: 304.12 K, C2H2: 308.3 K, CH4: 190.56 K) [33]; (d) The higher polarizability of CO2 and C2H2 than CH4 resulted in stronger interactions between the framework of 1a and CO2 or C2H2. All these factors contribute to the preferable adsorption of 1a toward CO2 and C2H2 to CH4 [34, 35].
In summary, a novel 3D MOF material 1 with 1D microporous was rationally designed and constructed by cross-linking 1D coordination polymer chains. The dense functional active sites on the inner walls of the channels can offer strong binding affinities to specific guest molecules, and thus favorable for the gas separation performance. Furthermore, gas adsorption experiments confirm that 1a have relatively high CO2 and C2H2 adsorption capacities over CH4 at 1 bar. The predicted IAST adsorption selectivities for CO2/CH4 and C2H2/CH4 are 10.4, 11.2 at 298 K and 12.5, 17.1 at 273 K. This work not only reported a porous MOF for effectively selective separation of CO2/CH4 and C2H2/CH4, but also opened a new perspective for the rational predesign and functionalization of MOFs materials.
Declaration of competing interestThe authors report no declarations of interest
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (NSFC, Nos. 91856124 and 21531005), Nature Science Fund of Tianjin, China (No. 19JCZDJC37200).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.09.014.
| [1] |
R.B. Lin, S. Xiang, H. Xing, et al., Coord. Chem. Rev. 378 (2019) 87-103. DOI:10.1016/j.ccr.2017.09.027 |
| [2] |
B. Li, H.M. Wen, Y. Yu, et al., Mater. Today Nano 2 (2018) 21-49. DOI:10.1016/j.mtnano.2018.09.003 |
| [3] |
J.R. Li, R.J. Kuppler, H.C. Zhou, Chem. Soc. Rev. 38 (2009) 1477-1504. DOI:10.1039/b802426j |
| [4] |
D.S. Sholl, R.P. Lively, Nature 532 (2016) 435-437. DOI:10.1038/532435a |
| [5] |
W. Fan, X. Liu, X. Wang, et al., Inorg. Chem. Front. 5 (2018) 2445-2449. DOI:10.1039/C8QI00652K |
| [6] |
X. Zhao, Y. Wang, D.S. Li, et al., Adv. Mater. 30 (2018) 1705189. DOI:10.1002/adma.201705189 |
| [7] |
J.W. Zhang, M.C. Hu, S.N. Li, et al., Chem. Commun. 54 (2018) 2012-2015. DOI:10.1039/C7CC09484A |
| [8] |
G. Si, X. Kong, T. He, et al., Chin. Chem. Lett. 32 (2021) 918-922. DOI:10.1016/j.cclet.2020.07.023 |
| [9] |
M.H. Yu, B. Space, D. Franz, et al., J. Am. Chem. Soc. 141 (2019) 17703-17712. DOI:10.1021/jacs.9b07807 |
| [10] |
J.J. Ma, W.S. Liu, Dalton Trans. 48 (2019) 12287-12295. DOI:10.1039/C9DT01907C |
| [11] |
W.P. Lustig, S. Mukherjee, N.D. Rudd, et al., Chem. Soc. Rev. 46 (2017) 3242-3285. DOI:10.1039/C6CS00930A |
| [12] |
Z.Q. Yao, J. Xu, B. Zou, et al., Angew. Chem. Int. Ed. 58 (2019) 5614-5618. DOI:10.1002/anie.201900190 |
| [13] |
Y. J.-Zhao, R. Wang, S. Wang, et al., J. Mater. Chem. A 7 (2019) 7389-7395. DOI:10.1039/C8TA12116H |
| [14] |
T. Zhang, W. Lin, Chem. Soc. Rev. 43 (2014) 5982-5993. DOI:10.1039/C4CS00103F |
| [15] |
J. Liu, L. Chen, H. Cui, et al., Chem. Soc. Rev. 43 (2014) 6011-6061. DOI:10.1039/C4CS00094C |
| [16] |
D. Bai, F. Chen, D. Jiang, Y. He, Inorg. Chem. Front. 4 (2017) 1501-1508. DOI:10.1039/C7QI00289K |
| [17] |
J.R. Li, J. Yu, W. Lu, et al., Nat. Commun. 4 (2013) 1538. DOI:10.1038/ncomms2552 |
| [18] |
B. Liu, S. Yao, C. Shi, et al., Chem. Commun. 52 (2016) 3223-3226. DOI:10.1039/C5CC09922F |
| [19] |
X. Wang, Y. Zhang, Z. Chang, et al., Chin. J. Chem. 37 (2019) 871-877. DOI:10.1002/cjoc.201900247 |
| [20] |
K. Liu, B. Ma, X. Guo, et al., CrystEngComm 17 (2015) 5054-5065. DOI:10.1039/C5CE00807G |
| [21] |
S.D. Burd, S. Ma, J.A. Perman, et al., J. Am. Chem. Soc. 134 (2012) 3663-3666. DOI:10.1021/ja211340t |
| [22] |
P. Nugent, Y. Belmabkhout, S.D. Burd, et al., Nature 495 (2013) 80-84. DOI:10.1038/nature11893 |
| [23] |
C.H. Springsteen, R.D. Sweeder, R.L. LaDuca, Cryst. Growth Des. 6 (2006) 2308-2314. DOI:10.1021/cg060229b |
| [24] |
V. Guillerm, Garzon-Tovar L., A. Yazdi, et al., Chem. -Eur. J. 23 (2017) 6829-6835. DOI:10.1002/chem.201605507 |
| [25] |
O'Nolan D., A. Kumar, M.J. Zaworotko, J. Am. Chem. Soc. 139 (2017) 8508-8513. DOI:10.1021/jacs.7b01682 |
| [26] |
V.A. Blatov, A.P. Shevchenko, D.M. Proserpio, Cryst. Growth Des. 14 (2014) 3576-3586. DOI:10.1021/cg500498k |
| [27] |
S. Brunauer, P.H. Emmett, E. Teller, J. Am. Chem. Soc. 60 (1938) 309-319. DOI:10.1021/ja01269a023 |
| [28] |
I. Langmuir, J. Am. Chem. Soc. 40 (1918) 1361-1403. DOI:10.1021/ja02242a004 |
| [29] |
O.T. Qazvini, R. Babarao, Z.L. Shi, et al., J. Am. Chem. Soc. 141 (2019) 5014-5020. DOI:10.1021/jacs.9b00913 |
| [30] |
M. Du, C.P. Li, M. Chen, et al., J. Am. Chem. Soc. 136 (2014) 10906-10909. DOI:10.1021/ja506357n |
| [31] |
A. Cadiau, Y. Belmabkhout, K. Adil, et al., Science 356 (2017) 731-735. DOI:10.1126/science.aam8310 |
| [32] |
H. Li, L. Li, R.B. Lin, et al., ACS Sustain. Chem. Eng. 7 (2019) 4897-4902. DOI:10.1021/acssuschemeng.8b05480 |
| [33] |
B.E. Poling, J.M. Prausnitz, J.P. O'Connell, The Properties of Gases and Liquids, 5th ed., McGraw-Hill, 2000.
|
| [34] |
F. Chen, Y. Wang, D. Bai, et al., J. Mater. Chem. A 6 (2018) 3471-3478. DOI:10.1039/C7TA10123F |
| [35] |
Y. Wang, M. He, X. Gao, et al., Dalton Trans. 47 (2018) 12702-12710. DOI:10.1039/C8DT02686F |
 2021, Vol. 32
2021, Vol. 32 

