In the past three decades, the formation and utilization of zeolite membranes has been drawn wide attention because of their homogeneous pore size, high thermal and mechanical stabilization. Up to now, zeolite LTA [1-4], FAU [5, 6], MFI [7, 8], MOR [9], CHA [10], DDR [11] membranes have been prepared for gases or liquids separations. Zeolite LTA membranes, with strong hydrophilicity and uniform pore size (~ 0.4 nm), are a promising candidate for dehydration of alcohols and other organics by pervaporation [12, 13]. Indeed, LTA membranes were installed at an industrial scale for removal of water from bio-ethanol [14]. However, due to the high negative surface charge of zeolite LTA building blocks, it is usually hard to gain a continuous LTA layer under charge repulsion [15].
Dozens of efforts have been made to assist to grow compact zeolite LTA membranes. Besides the secondary growth [12-14], preparation in the microwave field [2] or electric fields [16], there are a multitude of synthesis strategies through covalent modification of the substrates. Since both zeolite LTA blocks and alumina substrate have strong negative surface charge, organic polymers poly(diallyldimethylammonium chloride) (poly-DADMAC) are used to modify substrates surface to reverse its negative zeta potential [17, 18]. Therefore, LTA particles can facilely migrate to the positively charged substrates surface for the preparation of a uniform and compact LTA membrane. Recently, via chemical modification of the substrates by 3-aminopropyltriethoxysilane [19], 1, 4-diisocyanate [20], 3-chloropropyltrimethoxysilane [21], polydopamine [22], a "bridge" between the zeolite layer and the substrates can be built. Thus, zeolite precursors were adsorbed and anchored onto the substrates surface, which is helpful to enhance growth of a well-intergrown membrane. Nevertheless, the developed reagents suffers from high-cost, thus it is worth to explore other cheaper reagents to promote the growth of zeolite membranes.
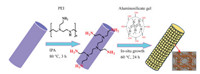
Polyethyleneimine (PEI) is widely utilized as a cross-linker for heavy metals removal [23] and CO2 adsorption [24] owing to its highly branched structure with a large quantity of -NH2 groups. As reported previously [25], covalent bonds between zeolite crystals and the substrates increase by introducing PEI as the linkers. In addition, it can be expected that hydrophilic LTA membranes tend to grow on the hydrophilic substrates after PEI modification followed by "like grows like" principle [26]. In this study, we report a facile method for growth of compact zeolite LTA membranes on the PEI-modified substrates (Fig. 1). The separation ability of the zeolite LTA membrane was evaluated for de-water of alcohols by pervaporation.
|
Download:
|
| Fig. 1. Scheme of seeding-free synthesis of zeolite LTA membranes on polyethyleneimine (PEI) modified substrates. | |
The LTA membranes were prepared on the PEI-modified substrates by hydrothermal synthesis (The details are shown in Supporting information). Fig. S1 (Supporting information) shows the FT-IR spectra of non-modified and PEI-modified α-Al2O3. Comparing with the FT-IR spectrum of the primitive α-Al2O3, the FT-IR spectrum of the PEI-modified α-Al2O3 contains bands at 1250, 1380, 1650 cm-1. A relatively board absorption band at 3420 cm-1 can be assigned to O-H stretching vibrations. Meanwhile, the spectrum of the PEI-modified α-Al2O3 shows a sharper O-H absorption than that of the α-Al2O3, suggesting that partial -OH groups have been reacted with N-H groups of PEI. The frequency absorption at 1650 cm-1 is related to N-H bending vibration. The lower absorptions observed at 1380 and 1250 cm-1 result from the stretching vibrations of C-N groups. The presence of N-H, C-N bands after modification with PEI indicates that PEI has been grafted on the surface of the α-Al2O3 substrate.
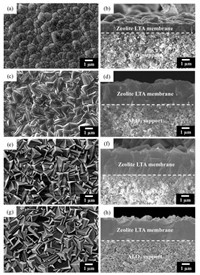
Fig. 2 shows FESEM images of the LTA membranes grown on the PEI-modified α-Al2O3 disks for different synthesis time at 60 ℃. As shown in Fig. 2a, the substrate surface has been covered by hemispherical and compactly-packed LTA crystals after crystallizing for 12 h, and a relatively homogeneous layer with a thickness of around 1.0 μm has formed (Fig. 2b). However, there are visible gaps among the zeolite LTA crystals (Fig. 2a). As time increases, the thicknesses of the zeolite LTA membranes successively increase to 1.8 μm (16 h, Fig. 2d), 2.8 μm (20 h, Fig. 2f), 3.0 μm (24 h, Fig. 2h), respectively. In a good agreement with the previous work [21], not only the size of the zeolite LTA crystals grow larger, but also the shape of the LTA crystals changes from sphere to cube (Figs. 2c, e and g). When the crystallization time is up to 24 h, the thickness of zeolite LTA membrane increases slightly in comparison with that prepared for 20 h, and the substrate surface has completely covered by homogenous and cubic LTA crystals, achieving a well intergrown LTA membrane, indicating the crystallization of zeolite LTA has accomplished within 24 h [20, 21]. However, no compact zeolite LTA membrane can be formed without modification, which is similar to the previous reports [19, 21], and observable defects is formed in the zeolite layer (Fig. S2a in Supporting information).
|
Download:
|
| Fig. 2. FESEM images of the zeolite LTA membrane prepared on PEI-modified α-Al2O3 disks for different synthesis time at 60 ℃: (a, b) 12 h, (c, d) 16h, (e, f) 20 h and (g, h) 24 h. (a, c, e and g) Top views, (b, d, f and h) cross-sections. | |
The development of FESEM images agrees with the results of XRD patterns (Fig. S3 in Supporting information). After 12 h growth, the phase of zeolite LTA has been detected together with α-Al2O3 substrate, showing no perfect orientation as LTA powder. When the crystallization time is more than 12 h, the intensity of the (222) peak becomes stronger and stronger, and the intensity of the (200) peak is remarkably reduced, suggesting that the (222) face of the zeolite LTA crystals are grown perpendicular to the substrate surface. In our previous reports [17, 21], (222) oriented zeolite LTA membranes were also formed on DADMAC and CPTMS modified substrates. Generally, the microcrystals tend to maximize their contact with the substrate surface to minimize the surface energy [27]. Probably, the zeolite LTA crystals prefer to grow with their C3 axis ([222]) vertical to the PEI-modified Al2O3 substrate. Further, besides the peaks of the α-Al2O3 substrate, all peaks of the XRD patterns conform to those of the zeolite LTA, suggesting that phase-pure zeolite LTA membranes have prepared on the PEI-modified α-Al2O3 surface.
For practical membrane separation, tubular membranes are more applicable for large-scale fabrication than flat ones. As shown in Figs. S4a and b (Supporting information), a well-intergrown zeolite LTA membrane with a thickness of about 6.0 μm is able to form on PEI-modified α-Al2O3 tubes. Moreover, compact zeolite LTA membranes are consistently prepared on various PEI-modified substrates, such as stainless steel nets (Fig. S5b in Supporting information), stainless steel disk (Fig. S5c in Supporting information) and PTFE disks (Fig. S5d in Supporting information), suggesting that PEI modification is advantageous to eliminate the negative effect of the substrates surface on the membrane growth. In addition, PEI modification also promotes facile formation of FAU membranes. No compact FAU membranes can grow on non-modified substrates (Fig. S2c in Supporting information), while dense ones can be prepared easily on the PEI-modified α-Al2O3 substrate (Fig. S2d in Supporting information). Further work is ongoing to prepare other molecular sieve membranes via PEI modification.
The separation performance of the zeolite LTA membrane prepared on PEI-modified α-Al2O3 tubes were tested for separation of alcohol/water by pervaporation. Fig. 3 shows the performances of the LTA membrane at different temperatures for the separation of 90 wt% IPA/water solution. In agreement with the previous report [12], the fluxes increase significantly with temperature, i.e., the flux of the zeolite LTA membrane enhances from 0.62 kg m-2h-1 to 1.73 kg m-2h-1 when the temperature rises from 45 ℃ to 90 ℃. With enhancement of operation temperature, the vapor pressure on the feed side increases, but that on the permeate side remains unchanged, which leads to an increasing of the driving force across the LTA membrane. Moreover, according to the thermodynamic principles, the diffusion rate of water molecules through membrane channel will accelerates with the increasing of temperature. Therefore, the flux increases with temperature. Meanwhile, the water/IPA separation factor of the membrane also increases as well, which achieves 44991 at 90 ℃, indicating that the LTA membrane performs high pervaporation capacity.

|
Download:
|
| Fig. 3. Pervaporation properties of the zeolite LTA membrane prepared on PEI-modified α-Al2O3 tube as a function of the operation temperature for the separation of 90 wt% iso-propanol/water mixture. | |
Fig. S6 (Supporting information) shows the performances of the LTA membrane at 90 ℃ for the separation of IPA/water solution with different IPA concentrations by pervaporation. As the IPA concentration in the feed ranges from 30 wt% to 90 wt%, the flux is found to change from 3.65 kg m−2h−1 to 1.73 kg m−2h−1 due to a diminution of water driving force [12], and water/IPA separation factors lessen from 44991 to 37491. It should be noted that the IPA concentration in the feed is still found less than 0.025% when the IPA concentration in feed is up to 90%. Such a high selectivity is mostly achieved owing to the high molecular selectivity of the LTA membrane, suggesting that the LTA membrane is nearly defect-free, or the non-zeolitic pore size is smaller than the size of zeolite channels. Moreover, the as-synthesized LTA membranes also show high separation capacity to separate other 90 wt% alcohols/water mixtures (Table S1 in Supporting information), and separation factors of 2991 and 37491 were achieved for dehydration of 90 wt% methanol/water and ethanol/water mixture, respectively. Meanwhile, Table S1 also shows the pervaporation performances of the zeolite LTA membranes reported previously. In comparison with literatures, the zeolite LTA membranes in the present work also display high separation performances.
In conclusion, we have proposed a facile synthesis strategy to synthesize compact zeolite LTA membranes on multifarious PEI-modified substrates. Thanks to the introduction of covalent linker, PEI plays a important role in strengthening bonding force between the LTA layer and substrate surface, which facilitate the growth of a thin, well-intergrown and phase-pure zeolite LTA membrane. The as-synthesized LTA membranes on the PEI-modified α-Al2O3 tubes perform high excellent performance for the separation of alcohols/water solution. At 90 ℃, a flux of 1.73 kg m−2h−1 and water/IPA separation factor of 44991 are achieved for the dehydration of 90 wt% IPA/water solution, which is promising for industrial requirements.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsFinancial supports by the National Natural Science Foundation of China (Nos. 21761132003, 21878100), and the Fundamental Research Funds for the Central Universities (No. 40500-20101-222093) are acknowledged.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.07.047.
| [1] |
K. Aoki, K. Kusakabe, S. Morooka, J. Membr. Sci. 141 (1998) 197-205. DOI:10.1016/S0376-7388(97)00300-1 |
| [2] |
X. Xu, W. Yang, J. Liu, L. Lin, Adv. Mater. 12 (2000) 195-198. DOI:10.1002/(SICI)1521-4095(200002)12:3<195::AID-ADMA195>3.0.CO;2-E |
| [3] |
Z. Wang, Q. Ge, J. Shao, Y. Yan, J. Am. Chem. Soc. 131 (2009) 6910-6911. DOI:10.1021/ja901626d |
| [4] |
C. Xu, C. Zhou, S. Wang, A. Huang, Chin. Chem. Lett. 30 (2019) 1204-1206. DOI:10.1016/j.cclet.2019.01.016 |
| [5] |
K. Kusakabe, T. Kuroda, A. Murata, S. Morooka, Ind. Eng. Chem. Res. 36 (1997) 649-655. DOI:10.1021/ie960519x |
| [6] |
A. Huang, N. Wang, J. Caro, J. Membr. Sci. 389 (2012) 272-279. DOI:10.1016/j.memsci.2011.10.036 |
| [7] |
J. Hedlund, M. Noack, P. Kolsch, D. Creaser, J. Caro, J. Sterte, J Membr. Sci. 159 (1999) 263-273. DOI:10.1016/S0376-7388(99)00069-1 |
| [8] |
Z. Lai, G. Bonilla, I. Diaz, et al., Science 300 (2003) 456-460. |
| [9] |
R. Zhou, Z. Hu, N. Hu, et al., Microporous Mesoporous Mater. 156 (2012) 166-170. DOI:10.1016/j.micromeso.2012.02.023 |
| [10] |
M. Carreon, S. Li, J. Falconer, R. Noble, J. Am. Chem. Soc. 130 (2008) 5412-5413. DOI:10.1021/ja801294f |
| [11] |
M. Kanezashi, J. O'Brien-Abraham, Y.S. Lin, K. Suzuki, AIChE J. 54 (2008) 1478-1486. DOI:10.1002/aic.11457 |
| [12] |
D. Shaha, K. Kissicka, A. Ghorpadeb, R. Hannahb, D. Bhattacharyya, J. Membr. Sci. 179 (2000) 185-205. DOI:10.1016/S0376-7388(00)00515-9 |
| [13] |
A. Huang, Y. Lin, W. Yang, J. Membr. Sci. 245 (2004) 41-51. DOI:10.1016/j.memsci.2004.08.001 |
| [14] |
Y. Morigami, M. Kondo, J. Abe, H. Kita, K. Okamoto, Sep. Purif. Technol. 25 (2001) 251-260. DOI:10.1016/S1383-5866(01)00109-5 |
| [15] |
J. Caro, D. Albrecht, M. Noack, Sep. Purif. Technol. 66 (2009) 143-147. DOI:10.1016/j.seppur.2008.11.009 |
| [16] |
A. Huang, W. Yang, Microporous Mesoporous Mater. 102 (2007) 58-69. DOI:10.1016/j.micromeso.2006.12.005 |
| [17] |
A. Huang, J. Caro, Chem. Mater. 22 (2010) 4353-4355. DOI:10.1021/cm1016189 |
| [18] |
X. Gong, J. Zhang, S. Jiang, Chem. Commun. 56 (2020) 3054-3057. DOI:10.1039/C9CC08768K |
| [19] |
A. Huang, F. Liang, F. Steinbach, J. Caro, J. Membr. Sci. 350 (2010) 5-9. DOI:10.1016/j.memsci.2009.12.029 |
| [20] |
A. Huang, J. Caro, J. Mater. Chem. 21 (2011) 11424-11429. DOI:10.1039/c1jm11549a |
| [21] |
A. Huang, Q. Liu, N. Wang, et al., J. Membr. Sci. 437 (2013) 57-64. DOI:10.1016/j.memsci.2013.02.058 |
| [22] |
C. Yuan, Q. Liu, H. Chen, A. Huang, RSC Adv. 4 (2014) 41982-41988. DOI:10.1039/C4RA05400H |
| [23] |
Y. Pang, G. Zeng, L. Tang, et al., Desalination 281 (2011) 278-284. DOI:10.1016/j.desal.2011.08.001 |
| [24] |
A. Huang, B. Feng, Int. J. Hydrogen. Energ. 43 (2018) 2224-2231. DOI:10.1016/j.ijhydene.2017.12.070 |
| [25] |
A. Kulak, Y.S. Park, Y.J. Lee, et al., J. Am. Chem. Soc. 122 (2000) 9308-9309. DOI:10.1021/ja001321d |
| [26] |
X. Wu, C. Liu, J. Caro, A. Huang, J. Membr. Sci. 559 (2018) 1-7. DOI:10.1016/j.memsci.2018.04.053 |
| [27] |
K. Yoon, Acc. Chem. Res. 40 (2007) 29-40. DOI:10.1021/ar000119c |
 2021, Vol. 32
2021, Vol. 32 

