b Key Laboratory of Hunan Province for Water Environment and Agriculture Product Safety, Changsha 410083, China;
c College of Biological Chemical Science and Engineering, Jiaxing University, Jiaxing 314001, China;
d College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou 450001, China
In the past decades, fluorescent sensing has become an efficient and powerful tool for biological applications because of its high sensitivity, good selectivity, high temporal-spatial resolution and simple technique operation [1-4]. The studies showed that homocysteine (Hcy), cysteine (Cys) and glutathione (GSH) are crucial in many physiological processes and their concentration variations can cause many diseases [5-8], such as Alzheimer's disease, renal diseases, heart diseases, cancer and slow growth in children. Particularly, they have close relationships between their metabolisms and biological functions. In order to understand the biologically interplaying roles of Hcy, Cys and GSH, it is of enormous importance to construct single-molecular fluorescent sensors that can distinguish them in vitro and in vivo.
Nitrobenzoxadiazole (NBD) group has been demonstrated as a perfect sensing group to distinguish Hcy/Cys from GSH in the construction of fluorescent probes [9-15]. In these NBD-based fluorescent probes, the NBD group and a fluorophore were coupled through an ether bond to form the probes and NBD group serves as a sensing group to quench the fluorophores' fluorescence as well as a precursor of a green fluorescent dye. To date, most of these reported NBD-based probes need two excitation wavelengthes (Tables S1 and S2 in Supporting information). Since NBD-based fluorescent probes are bifunctional to differentiate Hcy/Cys and GSH, there should be two sets of emission signals needed: one from the original fluorophore and the other from the produced green-emitting intermediate NBD-N-Hcy/Cys. Ideally, bifunctional fluorescent probes should have: 1) a single-wavelength excitation, which can avoid the need of double or multiple excitation sources and simplify the sensing process, and thereby remarkably improving the data accuracy for quantitative analysis [16-18]; 2) two well-separated and equally intensified fluorescence signals to avoid the interference. Since the green-emitting NBD-N-Hcy/Cys is certain, the performance of NBD-based fluorescent probes mainly relies on the optical properties of the paired fluorophores. It is preferred that the paired fluorophores for the design of NBD-based fluorescent probe should have the following optical properties: 1) an absorption possessing large spectral overlap with and similar molar absorptivity to those of NBD-N-Hcy/Cys (420−520 nm) to realize a single-wavelength excitation [19-21]; 2) a distinct emission spectrum from NBD-N-Hcy/Cys to obtain well-separated signals; 3) a similar fluorescent quantum yield to NBD-N-Hcy/Cys to obtain two emissions with comparable strengths; 4) red/NIR emission to deeply penetrate tissue, reduce photodamage to tissues and minimize autofluorescence; and 5) a large Stokes shift to avoid self-absorption and interference of the incident light [22, 23].
Very recently, we used tetrahydroquinoxaline (TQ) group to replace the diethylamino group in blue-emitting boron fluoride complexes to obtain a series of new fluorophores, TQBFs, which emit in the red spectral region (617−684 nm), possess really large Stokes shifts (up to 209 nm) and especially unexpected high fluorescence efficiencies (up to 0.68 in dichloromethane) [24]. In particular, the absorption maxima of TQBFs dyes are around 450−470 nm, which largely overlapped with the absorption of the green fluorescent intermediates, NBD-N-Hcy/Cys, of NBD-based fluorescent probes (absorption maxima is around 460−480 nm, Table S1) [21]. Inspired by the merits of the optical properties of TQBFs dyes, we are motivated to use them as the fluorophore to construct NBD-based fluorescent probes.
Herein, we first prepared a new boron fluoride fluorescent dye, TQBF−OH (Scheme 1). Next, we masked the OH group in dye TQBF−OH to offer a fluorescent probe, TQBF-NBD, which was sensitive and selective to distinguish Hcy/Cys from GSH with desired optical properties: single-wavelength excitation, red fluorescence and large Stokes shift.

|
Download:
|
| Scheme 1. Synthesis of TQBF-NBD. | |
As illustrated in Scheme 1, tetrahydroquinoxaline-derived salicylaldehyde and p-hydroxyl-aniline were refluxed in ethanol with p-toluenesulfonic acid as a catalyst to afford compound 1, which reacted with BF3∙OEt2 to give the boron fluoride complex, TQBF−OH. In addition, NBD-N-nBu was prepared as an analogue of NBD-N-Hcy/Cys, the green-emitting intermediates in NBD-based fluorescent probes (Scheme S1 in Supporting information).
To our delight, as shown in Fig. 1, dye TQBF−OH displayed an intense absorption with λmaxAbs=445 nm, a red fluorescence with λmaxEm=620 nm, a 175 nm Stokes shift and a desired fluorescent quantum yield (Φ=0.04 in PBS: 20% CH3CN, pH 7.4). First, there is a large overlap between the absorptions of TQBF−OH and NBD-N-nBu to allow a single-wavelength excitation, as well as the similar molar absorptivity of TQBF−OH (ε=24000 L mol−1cm−1) and NBD-N-nBu (ε = 22000 L mol−1cm−1) to guarantee matched photon absorption (Fig. 1). Second, there is a 70 nm difference between the maxima of the emissions of TQBF−OH (λmaxEm=620 nm) and NBD-N-nBu (λmaxEm=550 nm), which is large enough to separate these two signals. Third, the fluorescent quantum yields of TQBF−OH (Φ = 0.04) and NBD-N-nBu (Φ = 0.05) [25] are very close, ensuring the green and red fluorescence signals have a comparable intensity. Therefore, the photophysical properties of TQBF−OH perfectly satisfied the requirements of optical properties of ideal fluorophores for NBD-based fluorescent probes.

|
Download:
|
| Fig. 1. Comparison of photophysical properties of TQBF−OH and NBD-N-nBu in PBS (0.01 mol/L, pH 7.4, 20% CH3CN). (A) Normalized absorption spectra. (B) Normalized emission spectra. (C) Fluorescent quantum yields. | |
The advantageous optical properties of TQBF−OH encourage us to prepare the fluorescent probe TQBF-NBD by refluxing TQBF−OH with 4-chloro-7-nitro-2, 1, 3-benzoxadiazole (NBD-Cl) in acetonitrile using triethylamine as a base (Scheme S1).
The proposed sensing mechanism is shown in Scheme 2. We expected that the fluorescence of TQBF-NBD would be strongly quenched by NBD group via photo-induced electron transfer (PET). In the presence of Hcy, Cys and GSH, the ether bond of probe would be cleaved to release the red-emitting TQBF−OH and the non-emissive intermediates NBD-S-Hcy/Cys/GSH. Subsequently, NBD-S-Hcy/Cys can fast proceed an intermolecular Smiles rearrangement to generate green-emitting compounds NBD-N-Hcy/Cys. It is an exception for NBD-S-GSH that 10-membered cyclization reaction would be kinetically inhibited. As a result, the probe is expected to respond to Hcy/Cys to produce mixed red and green signals, whereas only give red fluorescence in response to GSH.

|
Download:
|
| Scheme 2. Reaction mechanism of TQBF-NBD toward Hcy, Cys and GSH. | |
As expected, TQBF-NBD was non-emissive in PBS (0.01 mol/L, pH 7.4, 20% CH3CN) (Fig. 2). By contrast, the presence of Hcy/Cys (10.0 equiv.) to TQBF-NBD triggered strong fluorescence with two maxima at 550 nm and 620 nm, which should be from the green-emitting NBD-N-Hcy/Cys and the red-emitting TQBF−OH. As for GSH, only strong red fluorescence signals were seen with a λmax=620 nm.

|
Download:
|
| Fig. 2. Emission spectra of TQBF-NBD (10.0 μmol/L) in PBS (0.01 mol/L, pH 7.4, 20% CH3CN), upon the addition of related amino acids (Hcy, Cys, GSH, Ala, Glu, Asp, Gly, His, Iso, Arg, Leu, Thr, Trp, Met, Lys, Phe, Ser, Val, Tyr). Incubation time: 20 min. Excitation wavelength: 450 nm. | |
In order to inspect the selectivity of TQBF-NBD, other biologically relevant amino acids (Ala, Glu, Gly, Arg, Iso, Leu, Asp, His, Lys, Thr, Met, Phe, Ser, Trp, Val, Tyr, 10.0 equiv.) were used. As seen in Fig. 2, these interfering species hardly caused any fluorescence changes. Moreover, the coexistence of other amino acids did not disturb the detection performance of probe for Hcy/Cys and GSH. As shown in Fig. 3, the mixtures of TQBF-NBD with other interfering species hardly produced fluorescence enhancement at 620 nm. However, the additional addition of Hcy/Cys/GSH to the above mixtures induced about 200-fold enhancements.

|
Download:
|
| Fig. 3. The relative fluorescence enhancement (620 nm) of the mixtures of TQBF-NBD with the co-existence of other respective amino acids in PBS (0.01 mol/L, pH 7.4, 20% CH3CN) in the presence (red bars) and absence (green bars) of Cys (A), Hcy (B), GSH (C), respectively. Incubation time: 20 min. Excitation wavelength: 450 nm. | |
Thus, it could be concluded that TQBF-NBD could detect Hcy/Cys and GSH with good selectivity and anti-interference.
To confirm whether TQBF-NBD is suitable for quantitative analysis, the fluorescence intensities of TQBF-NBD with the addition of varied amounts of Hcy, Cys and GSH (0–30.0 equiv.) were determined individually. As seen in Fig. 4, the fluorescence signals at both 550 nm and 620 nm of TQBF-NBD were progressively intensified as the concentration of Cys or Hcy increased and were saturated when the concentration was over 200.0 μmol/L (20.0 equiv.). By observing the fluorescence at 620 nm, we obtained similar results for GSH and the saturation concentration is 250.0 μmol/L (25.0 equiv.). We were pleased that TQBF-NBD could quantitatively detect Hcy, Cys and GSH with good linearities in a concentration range of 0−100 μmol/L (0.0–10.0 equiv.). The detection limits of TQBF-NBD were calculated to be 0.202, 0.359 and 0.228 μmol/L for Cys, Hcy and GSH, respectively, indicating that TQBF-NBD was highly sensitive.

|
Download:
|
| Fig. 4. (A1, B1, C1) Emission spectra of TQBF-NBD (10.0 μmol/L) with different concentration of thiols (A1: Cys; B1: Hcy; C1: GSH), respectively. (A2, B2, C2) Plots of fluorescence intensity of TQBF-NBD at 620 nm (10.0 μmol/L) against the concentration of thiols (A2: Cys; B2: Hcy; C2: GSH); insets: the linear relationship between the fluorescence intensity and the concentration of thiols. Excitation wavelength: 450 nm. | |
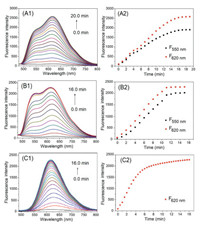
In order to determine the optimal response times of TQBF-NBD toward biothiols in PBS (0.01 mol/L, pH 7.4, 20% CH3CN), time-dependence experiments were carried out (Fig. 5). It is indicated that the reaction between TQBF-NBD (10.0 μmol/L) and 250.0 μmol/L (25.0 equiv.) of Cys was completed within 8 min. And the response times for GSH and Hcy were found to be 8 min and 15 min, respectively. Therefore, the fast responses of TQBF-NBD toward Hcy, Cys and GSH suggested that it held a good practicability in biological samples.
|
Download:
|
| Fig. 5. Time-dependent fluorescence behaviors of TQBF-NBD (10.0 μmol/L) with 250.0 μmol/L of Cys (A1, A2), Hcy (B1, B2) and GSH (C1, C2). (A1-C1) Emission spectra. (A2-C2) Plots of fluorescence intensities versus time. Excitation wavelength: 450 nm. | |
The fluorescence studies of TQBF-NBD well-matched our hypothesis on the sensing mechanism illustrated in Scheme 2. To provide decisive evidence, the mixtures of TQBF-NBD with these three biothiols were analysed by high-resolution mass spectrometry (Figs. S1-S3 in Supporting information). In the HRMS chromatograms (Fig. S1), we observed all the expected peaks for the corresponding reaction products: TQBF−OH (m/z for [M–H]: calcd. 372.1770; found: 372.1761, 372.1745, 372.1735) (Figs. S1-S3), NBD-N-Cys (m/z for [M–H]: calcd. 283.0215; found: 283.0194) (Fig. S1), NBD-N-Hcy (m/z for [M–H]: calcd. 297.0369; found: 297.0338) (Fig. S2) and NBD-S-GSH (m/z for [M–H]: calcd. 469.0853; found: 469.0819) (Fig. S3), respectively. For example, the HRMS chromatogram of TQBF-NBD with Cys displayed two peaks at 372.1761 and at 283.0194, which were assigned for the products of TQBF−OH (m/z for [M−H]: 372.1770) and NBD-N-Cys (m/z for [M−H]: 283.0215). The HMRS analysis forcefully confirmed the hypothesized reaction mechanism.
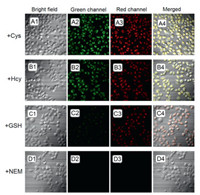
We next explored the practical application of TQBF-NBD in biological system. The cytotoxicity assays performed in living Hela cells showed TQBF-NBD was non-toxic, as shown in Fig. S4 (Supporting information). Using TQBF-NBD as a tool, the visualization of these three thiols was conducted in Hela cells (Fig. 6). Cells were first treated with N-ethylmaleimide (NEM, 1.0 mmol/L), a thiol-quenching agent, at 37 ℃ for 0.5 h. As expected, no fluorescence signal was seen when NEM-treated cells were incubated with TQBF-NBD for 0.5 h (Fig. 6D). When NEM-treated cells were cultured with 5.0 mmol/L Cys or Hcy for 0.5 h, then incubated with TQBF-NBD for an additional 0.5 h, bright fluorescence from both red and green channels were observed (Figs. 6A and B). And the successive treatment of NEM-treated cells with GSH and TQBF-NBD produced strong red fluorescence signals (Fig. 6C).
|
Download:
|
| Fig. 6. Images of cells. NEM-treated cells grown with 5.0 mmol/L of respective thiols for 0.5 h, respectively, and TQBF-NBD (10.0 μmol/L) for another 0.5 h. A: Cys; B: Hcy; C: GSH; D: blank. | |
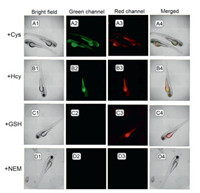
Moreover, the visualization of intra-organismal thiols in zebrafish was performed. As seen in Fig. 7, the results are as we expected: Mixed green and red fluorescence for Hcy/Cys (Figs. 7A and B) and red fluorescence for GSH (Fig. 7C).
|
Download:
|
| Fig. 7. Images of zebrafish. Zebrafish fed with NEM (10.0 mmol/L) for 0.5 h, cultured respectively with 5.0 mmol/L of Cys (A), Hcy (B), GSH (C) and blank (D) for another 0.5 h at 28 ℃, and finally stained with TQBF-NBD (10.0 μmol/L) for additional 0.5 h. | |
In conclusion, a rapid fluorescent probe, TQBF-NBD, was constructed to selectively and sensitively detect Hcy/Cys from GSH under a single light source based on a new red-emissive boron fluoride complex with a large Stokes shift. Also, this probe was successfully applied to distinguish Hcy/Cys from GSH in cells and zebrafish.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentWe thank the support from the National Natural Science Foundation of China (No. U1608222).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.09.024.
| [1] |
X. Chen, F. Wang, J.Y. Hyun, et al., Chem. Soc. Rev. 45 (2016) 2976-3016. DOI:10.1039/C6CS00192K |
| [2] |
H.N. Kim, M.H. Lee, H.J. Kim, et al., Chem. Soc. Rev. 37 (2008) 1465-1472. DOI:10.1039/b802497a |
| [3] |
L.Y. Niu, Y.Z. Chen, H.R. Zheng, et al., Chem. Soc. Rev. 44 (2015) 6143-6160. DOI:10.1039/C5CS00152H |
| [4] |
X. Jiao, Y. Li, J. Niu, et al., Anal. Chem. 90 (2018) 533-555. DOI:10.1021/acs.analchem.7b04234 |
| [5] |
S. Saharan, P.K. Mandal, J. Alzheimers Dis. 40 (2014) 519-529. DOI:10.3233/JAD-132483 |
| [6] |
C.C. Wu, C.M. Zheng, Y.F. Lin, et al., Clin. Biochem. 45 (2012) 1286-1294. DOI:10.1016/j.clinbiochem.2012.05.031 |
| [7] |
M.C. Miniaci, C. Irace, A. Capuozzo, et al., J. Cell. Biochem. 117 (2016) 402-412. DOI:10.1002/jcb.25286 |
| [8] |
H.L. Martin, P. Teismann, FASEB J. 23 (2009) 3263-3272. DOI:10.1096/fj.08-125443 |
| [9] |
P. Wang, Y. Wang, N. Li, et al., Sens. Actuators B-Chem. 245 (2017) 297-304. DOI:10.1016/j.snb.2017.01.127 |
| [10] |
J. Zhang, X. Ji, J.L. Zhou, et al., Sens. Actuators B-Chem. 257 (2018) 1076-1082. DOI:10.1016/j.snb.2017.10.133 |
| [11] |
L. He, X. Yang, K. Xu, et al., Chem. Comm. 53 (2017) 13168-13171. DOI:10.1039/C7CC07296A |
| [12] |
L.A. Montoya, T.F. Pearce, R.J. Hansen, et al., J. Org. Chem. 78 (2013) 6550-6557. DOI:10.1021/jo4008095 |
| [13] |
L. He, X. Yang, K. Xu, et al., Chem. Sci. 8 (2017) 6257-6265. DOI:10.1039/C7SC00423K |
| [14] |
Y.F. Kang, L.Y. Niu, Q.Z. Yang, Chin. Chem. Lett. 30 (2019) 1791-1798. DOI:10.1016/j.cclet.2019.08.013 |
| [15] |
L. Song, L.M. Ma, Q. Sun, et al., Chin. Chem. Lett. 27 (2016) 330-334. DOI:10.1016/j.cclet.2015.12.012 |
| [16] |
H.C. Zhu, C.Y. Liu, R.F. Yuan, et al., Analyst 144 (2019) 4258-4265. DOI:10.1039/C9AN00818G |
| [17] |
F. Hu, X.L. Cai, P.N. Manghnani, et al., Chem. Sci. 9 (2018) 2756-2761. DOI:10.1039/C7SC04585A |
| [18] |
K.X. Zhao, X. Z, H.J. Zhang, et al., J. Alloys. Compd. 793 (2019) 613-619. DOI:10.1016/j.jallcom.2019.04.146 |
| [19] |
S.S. Ding, G.Q. Feng, Sens. Actuators B-Chem. 235 (2016) 691-697. DOI:10.1016/j.snb.2016.05.146 |
| [20] |
H.J. Xiang, H.P. Tham, M.D. Nguyen, et al., Chem. Comm. 53 (2017) 5220-5223. DOI:10.1039/C7CC01814B |
| [21] |
W. Chen, H. Luo, X. Liu, et al., Anal. Chem. 88 (2016) 3638-3646. DOI:10.1021/acs.analchem.5b04333 |
| [22] |
T.B. Ren, W. Xu, W. Zhang, et al., J. Am. Chem. Soc. 140 (2018) 7716-7722. DOI:10.1021/jacs.8b04404 |
| [23] |
L. Yang, H.Q. Xiong, Y.A. Su, et al., Chin. Chem. Lett. 30 (2019) 563-565. DOI:10.1016/j.cclet.2018.12.017 |
| [24] |
X.J. Ren, F. Zhang, H.C. Luo, et al., Chem. Comm. 56 (2020) 2159-2162. DOI:10.1039/C9CC09523C |
| [25] |
X.J. Ren, H.H. Tian, L. Yang, et al., Sens. Actuators B-Chem. 273 (2018) 1170-1178. DOI:10.1016/j.snb.2018.04.163 |
 2021, Vol. 32
2021, Vol. 32 

