b Tianjin Key Laboratory of Biomedical Materials, Key Laboratory of Biomaterials and Nanotechnology for Cancer Immunotherapy, Institute of Biomedical Engineering, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 300192, China
Membrane separation technology belongs to the high-tech category, which has the characteristics of simple operation, energy saving, high efficiency and high safety [1-4]. It is one of the most promising technologies in the fields of water treatment, desalination, drug separation and protein purification [2, 5]. Due to its high separation efficiency, it has been widely used in the past few decades. Among them, the nanofiltration membrane allows inorganic salts and small molecules to pass through and repel large molecules, thereby achieving efficient separation of dye molecules and inorganic salts, and purification of drugs.
However, most nanofiltration membranes are made of hydrophobic polymer materials, such as PVDF, PAN, PSF [[6-8]. Particles, colloidal particles or solute macromolecules in water can easily block the pores and cause membrane fouling, thus limiting the applications of nanofiltration separation technology [9]. In order to overcome this shortcoming, people are exploring a variety of methods to modify the membrane material to give it certain characteristics, such as hydrophilicity, anti-pollution, self-cleaning, photocatalytic and photodegradability [10, 11]. Improving the hydrophilicity of membrane materials is an effective way to reduce membrane fouling. As a hydrophilic polymer material, polyvinyl alcohol, polyacrylic acid, chitosan, etc. can be used for membrane synthesis by coating, grafting and other methods to reduce membrane fouling [12, 13].
Sodium alginate is a natural polymer material extracted from kelp or brown algae. It has a wide range of sources, low prices and no toxicity, so it is widely used in food processing, biomedicine, water treatment and other fields [14, 15]. Sodium alginate and calcium ions form a hydrogel through ion cross-linking, and have high hydrophilicity because of a large number of hydrophilic groups (−OH, −COO−) in the molecular structure. In the previous work, Zhao et al. [16] prepared a self-supporting antifouling calcium alginate film by adding porogen, which has good retention of dye molecules. After three cycles of bovine serum albumin (BSA) and pure water alternating filtration, the flux recovery rate was as high as 90%. Due to the unique structure, the calcium alginate hydrogel membrane can achieve the fine separation of body molecules with similar structure and different molecular weights. But calcium alginate hydrogel is not antibacterial and the hydrogel can swell in monovalent ion solutions.
In this paper, copper ion doped calcium alginate (Cu2+/CaAlg) hydrogel antibacterial filter membrane was prepared by a simple ion crosslinking method. The Cu2+/CaAlg membrane were characterized by FTIR, water contact angle and thermal stability. A series of salts (MgCl2, MgSO4, NaCl and Na2SO4, 0.5 g/L) and dyes (coomassie brilliant blue G250 (CBB), direct black 38 (DB), Congo red (CR), amaranth (AR) and methyl orange (MO), 0.1 g/L) aqueous solutions were used for filtration tests to evaluate the membrane's permeability. The antibacterial performance and anti-fouling property of the obtained film were investigated. This membrane has potential application prospects in the separation of dyes, saline wastewater and purification of active ingredients of Chinese herbal medicine.
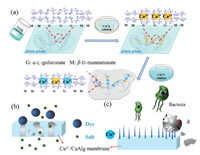
Fig. 1 shows the schematic representations for the preparation of Cu2+/CaAlg hydrogel filtration membrane, the dye/salt separation process and Cu2+ antibacterial principle. First, 2.5 wt% NaAlg aqueous solution was prepared. After vacuum defoaming, it is evenly scraped on a clean glass plate with a glass rod (winding a 0.4 mm diameter brass wire at both ends). Then put the glass plate in 2.5 wt% CaCl2 solution for cross-linking for 8 h to obtain CaAlg hydrogel membrane [16]. The Cu2+/CaAlg hydrogel filtration membrane was obtained by immersing the CaAlg membrane to a CuCl2 solution for 2 h to introduce Cu2+. The resulted Cu2+/CaAlg membrane was stored in deionized water before any treatment or test.
|
Download:
|
| Fig. 1. Schematic representation for the preparation of Cu2+/CaAlg hydrogel filtration membrane (a), dye/salt separation process (b) and antibacterial principle (c). | |
The flux and rejection rate of the Cu2+/CaAlg hydrogel filtration membrane is evaluated by a cross-flow filtration device. Yeast aqueous solution (0.5 g/L) was used to investigate the anti-fouling property of the membrane. CBB, DB, CR, AR, MO aqueous solutions (0.1 g/L) were used to research the rejection property of the Cu2+/CaAlg membrane. The chemical structures and maximum adsorption wavelength of the dyes are listed in Table S1 (Supporting information). The concentrations of dyes in the feed and permeate solutions were measured by UV–vis spectrophotometer (TU-1901, Beijing Purkinje, China). The flux (J, L m−2h−1) and the rejection (R, %) were calculated by following equations (Eqs. 1 and 2) [16]:
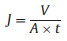
|
(1) |

|
(2) |
where V is the permeate volume (L), A is the effective membrane area (m2), t is the filtration time (h), Cp and Cf are the dye concentrations of permeate and feed solution, respectively.
The pure water flux (PWF) of Cu2+/CaAlg filtration membrane was determined at 0.1 MPa and denoted as Jwo (L m−2h−1). Then, the feed solution was switched to yeast solution for 60 min, and the flux of yeast solution was denoted as JB1. The process was repeated for three times. The anti-fouling property of the Cu2+/CaAlg membrane was determined by the flux recovery rate (FRR) (Eq. 3) [16]:
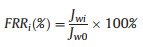
|
(3) |
where, Jwi is the PWF of i cycle.
Fourier transform infrared spectra (FTIR) of CaAlg and Cu2+/CaAlg membrane were measured. It can be seen from Fig. 2a that the C−OH stretching vibration absorption peak of the CaAlg filtration membrane is located at 1027.3 cm−1, and the Cu2+/CaAlg membrane has a weak absorption peak here. This is because the oxygen atom of the C−OH group in CaAlg forms a coordination structure with Ca2+, while the oxygen atom of the C−OH group does not participate in the coordination structure of Cu2+ [17]. The peak at 1414.2 cm−1 was assigned to the symmetric stretching peaks of −COO− groups, and 1583.6 cm−1 peak was corresponding to hydroxyl symmetric stretching. With respect to CaAlg membrane, the decrease of the distance between the two carboxyl bands of Cu2+/CaAlg membrane is an indication of complexation reactions between those groups and Cu2+. In addition, it can be seen from the DTG curves (Fig. 2b) that the introduced Cu2+ changes the structure of calcium alginate and changes its thermal cracking process.

|
Download:
|
| Fig. 2. FTIR spectra (a) and DTG curves (b) of CaAlg and Cu2+/CaAlg filtration membranes. The elastic modulus (c) and fracture energy (d) of different Cu2+/CaAlg membranes. (e) Swelling radio of different Cu2+/CaAlg membranes in 0.9 wt% NaCl solution. (f) Static water contact angle. | |
Figs. 2c and d show the mechanical properties of different Cu2+/CaAlg filter membranes. After doping with copper ions, the fracture energy and elastic modulus of the CaAlg film increased significantly. When the concentration of CuCl2 is 0.5 wt%, the mechanical strength of Cu2+/CaAlg film is the largest. As can be seen from Fig. 2e, compared with the CaAlg membrane, the anti-swelling performance of Cu2+/CaAlg membrane is significantly improved. When CuCl2 concentration was 2 wt%, the swelling rate of the membrane was the lowest, and swelling hardly occurred.Fig. 2f shows the static water contact angle of different Cu2+/CaAlg membrane. The stable water contact angles of CaAlg membrane and Cu2+/CaAlg membrane (1 wt% CuCl2) were 0.4° and 7.7°, respectively. The contact angle of the membrane decreased slightly after copper ion crosslinking, still below 10°, indicating that the Cu2+/CaAlg membrane has good hydrophilicity.
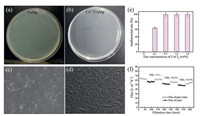
The number of colonies growing on the CaAlg membrane is 134 (Fig. 3a), while the Cu2+/CaAlg hydrogel membrane has no bacteria, and the antibacterial rate is 100% (Fig. 3b). After the CaAlg hydrogel membrane was stored in the deionized water for two weeks at room temperature, many microorganisms grew on the surface, as shown in Fig. 3c. The CaAlg hydrogel membrane does not show antibacterial property. Similarly, the SEM image of Cu2+/CaAlg hydrogel filtration membrane is shown in Fig. 3d. The Cu2+/CaAlg membrane showed good antibacterial property. Moreover, as shown in Fig. 3e, the antibacterial rate of the membrane increases with the increase of the CuCl2 concentration and the antibacterial rate reach 100% when the concentration of CuCl2 is 0.5 wt%. In addition, the surface SEM image shows the rough structure of the Cu2+/CaAlg membrane surface, while the surface of the CaAlg hydrogel membrane is relatively flat and smooth. Fig. 3f shows the antifouling properties of Cu2+/CaAlg membrane. The Cu2+/CaAlg membrane exhibits excellent yeast anti-fouling property. The FRR1 was 97.6%. After three consecutive yeast suspension filtrations, the FRR3 still reached 85.0% without any washing operation.
|
Download:
|
| Fig. 3. Demonstrated antibacterial properties of the membranes by the plate counting method: CaAlg (a), Cu2+/CaAlg membrane (b), FE-SEM images of CaAlg (c) and Cu2+/CaAlg membrane (d). (e) Antibacterial rate of Cu2+/CaAlg membranes prepared with different CuCl2 concentrations. (f) Alternating filtration flux between pure water and yeast solution. | |
Fig. 4a shows the flux and rejection of five different molecular weight dyes. The Cu2+/CaAlg membrane showed similar and high permeate fluxes (> 40 L m−2h−1bar−1) for almost all the dye solutions, but different rejection behaviors. The order of rejections is well in agreement with the order of the dye molecular weights. A rejection of 25.3% are obtained for the MO dye which has the smallest molecular weight of 327 Da among the dyes used in this study. The rejection goes up to 99.3% and 99.7% for DB and CBB dyes, respectively, which possess larger molecular weights. In addition, the dye molecules may form larger clusters or aggregates via hydrophobic interactions between their aromatic rings [18, 19]. Therefore, this Cu2+/CaAlg membrane has a high rejection rate for dyes with a molecular weight greater than 600 Da, and can be applied to fine separation of drugs and biological samples.

|
Download:
|
| Fig. 4. The flux and rejection of the Cu2+/CaAlg membranes to different dyes (a) and different salts (b). (c) Long-term performance of the Cu2+/CaAlg membrane. (d) Comparison of the dye removal performances of Cu2+/CaAlg membrane with other membranes reported in literatures. | |
Fig. 4b shows the flux and retention of Cu2+/CaAlg hydrogel filter membrane for CBB in different kinds of salt solutions. The rejection of the Cu2+/CaAlg membranes for different inorganic salts followed the order: Na2SO4 > MgSO4 > NaCl > MgCl2. The separation performance of the Cu2+/CaAlg membrane for different salts could be explained by sieving and dielectric exclusion effects. The rejection of salts was less than 10% because the ionic radius of salt is small and the salts can pass through the hydrogel film.
For practical application, the membrane stability under long-term operation is crucial [20]. The stability of the Cu2+/CaAlg membrane in the removal of CBB with 0.5 g/L Na2SO4 aqueous solution was investigated at room temperature. As shown in Fig. 4c, no obvious decrease in the permeation flux (ca. 46 L m−2 h−1 bar−1) was observed over 24 h and the rejection rate for CBB remained constant (ca. 100%). Cu2+/CaAlg membranes have a good long-term running stability. Therefore, this membrane exhibited excellent ability to fractionate and recovery valuable inorganic salts and dyes in textile wastewater.
The pure water flux can reach 61.4 L m−2 h−1 at 0.1 MPa. Compared with the conventional NF membranes and other reported NF membranes, the Cu2+/CaAlg membrane prepared at optimal conditions in present work exhibited relatively higher permeate flux and higher dyes rejection (Fig. 4d). The Cu2+/CaAlg membrane has broad application prospects in the fields of fine separation, dye desalination and wastewater treatment.
In summary, the Cu2+/CaAlg membrane provides a facile approach to constructing an efficient separation membrane with anti-fouling performance in dye desalination field.
Declaration of competing interestAll authors declare that they have no financial and personal relationships with other people or organizations that can inappropriately influence their work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
AcknowledgmentsThe research is supported by National Natural Science Foundation of China (Nos. 51678409, 51708406, 51708407), Tianjin Science Technology Research Funds of China (Nos. 16JCZDJC37500, 15JCZDJC38300), and Tianjin Science and Technology Plan Project (No. 18ZXJMTG00120).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.07.034.
| [1] |
X. Wang, Y. Pan, H. Yuan, et al., Chin. Chem. Lett. 31 (2020) 365-368. DOI:10.1016/j.cclet.2019.07.044 |
| [2] |
J. Shan, W. Wei, D. Yonghong, et al., Chin. Chem. Lett. 29 (2018) 390-394. DOI:10.1016/j.cclet.2018.01.006 |
| [3] |
S. Yuan, G. Zhang, J. Zhu, J. Mater. Chem. A 8 (2020) 3238-3245. DOI:10.1039/C9TA12984G |
| [4] |
Q. Nie, F. Ran, C. He, et al., Chin. Chem. Lett. 22 (2011) 370-373. DOI:10.1016/j.cclet.2010.10.017 |
| [5] |
J. Guo, K. Zhao, X. Zhang, et al., Mater. Lett. 157 (2015) 112-115. DOI:10.1016/j.matlet.2015.05.080 |
| [6] |
J. Li, S. Li, X. Wang, et al., Chin. Chem. Lett. 30 (2019) 239-242. DOI:10.1016/j.cclet.2018.07.016 |
| [7] |
J. Zhu, M. Tian, J. Hou, et al., J. Mater. Chem. A 4 (2016) 1980-1990. DOI:10.1039/C5TA08024J |
| [8] |
S. Gao, Y. Zhu, Y. Gong, et al., ACS Nano 13 (2019) 5278-5290. DOI:10.1021/acsnano.8b09761 |
| [9] |
P. Zhong, N. Widjojo, T. Chung, et al., J. Membr. Sci. 417 (2012) 52-60. |
| [10] |
J.H. Jhaveri, Z.V.P. Murthy, Desalination 379 (2016) 137-154. DOI:10.1016/j.desal.2015.11.009 |
| [11] |
M. Min, Y. Liu, C. Song, et al., ACS Appl. Mater. Inter. 10 (2018) 21246-21253. DOI:10.1021/acsami.8b03411 |
| [12] |
X. Deng, N. Chao, W. Ding, et al., J. Biomed. Nanotechnol. 15 (2019) 756-768. DOI:10.1166/jbn.2019.2713 |
| [13] |
Y. Cai, J. Zhang, Y. He, et al., Int. J. Biomed. Nanotechnol. 14 (2018) 257-266. DOI:10.1166/jbn.2018.2483 |
| [14] |
S. Gao, S.Y. Zhu, J. Wang, et al., Adv. Funct. Mater. 28 (2018) 1801944. DOI:10.1002/adfm.201801944 |
| [15] |
E.G. Deze, S.K. Papageorgiou, E.P. Favvas, et al., Chem. Eng. J. 209 (2012) 537-546. DOI:10.1016/j.cej.2012.07.133 |
| [16] |
K. Zhao, X. Zhang, J. Wei, et al., J. Membr. Sci. 492 (2015) 536-546. DOI:10.1016/j.memsci.2015.05.075 |
| [17] |
Y.N. Mata, M.L. Blázquez, A. Ballester, et al., J. Hazard. Mater. 163 (2009) 555-562. DOI:10.1016/j.jhazmat.2008.07.015 |
| [18] |
L.D. Nghiem, A.I. Schäfer, M. Elimelech, et al., J. Membr. Sci. 286 (2006) 52-59. DOI:10.1016/j.memsci.2006.09.011 |
| [19] |
W. Ye, K. Ye, F. Lin, et al., Chem. Eng. J. 379 (2020) 122321. DOI:10.1016/j.cej.2019.122321 |
| [20] |
N. Wang, X. Li, L. Wang, et al., ACS Appl. Mater. Interfaces 8 (2016) 21979-21983. DOI:10.1021/acsami.6b08581 |
 2021, Vol. 32
2021, Vol. 32 

