Nanoparticles are of great importance for biomedical applications, including drug delivery and bioimaging [1, 2]. Surface property of nanoparticles is considered to be the deciding factor for biophysicochemical interactions at the nano-bio interface [3]. Manipulation of nanoparticle surface characteristics may influence circulation stability and cell uptake efficiency [4-6]. To improve stability and avoid nonspecific adsorption of serum proteins or other biomolecules during circulation, nanoparticles are often coated with polyethylene glycol (PEG) or other "nonfouling" biomaterials to achieve "stealth" [7]. When reaching tumor or other target tissues, it is desired to enhance cell uptake and tissue accumulation [8]. Stimuli-responsive "sheddable" shell of PEG or other nonfouling coating material has been used to achieve the goal of cell uptake enhancement.
Although widely used, PEG suffers from limitations, such as susceptibility to oxidation damage and anti-PEG antibodies [9, 10]. Zwitterionic polymeric materials, such as poly(carboxybetaine), are second generation nonfouling biomaterials, which demonstrate ultralow-fouling toward serum proteins [11-13]. To render PEG- or zwitterionic polymer-coated nanoparticles stimuli-responsive, relatively complex design is required. For example, nonfouling and "charge-conversion" polymers have been used to achieve stimuli-responsive enhancement of cell uptake [14-16]. It is highly desired to develop a simple strategy to simultaneously ensure high stability in blood serum, and stimuli-induced cell uptake improvement in target tissues.
Host-guest interaction is a non-covalent force to conveniently assemble complex nanostructures with multistimuli-responsiveness [17-26]. We decided to explore the application of pillar[n] arene (PA[n])-based zwitterionic host molecules as nonfouling coating materials [27-35]. Gold nanoparticles (GNPs) were used as model nanoparticles, and nanoparticle surface was modified with azobenzene-based guest. Zwitterionic PA[n] (ZPn) was used to coat GNPs via host-guest interaction [36]. The resulting GNPs were discovered to be ultra-stable in blood serum. UV-irradiation, competitive displacement or acidic pH was used to manipulate surface property of GNPs by the removal of ZPn-coating layer or conversion of surface charge. Stimuli-induced conversion of GNP surface characteristics was confirmed to significantly enhance GNP uptake by human cancer cells in vitro.
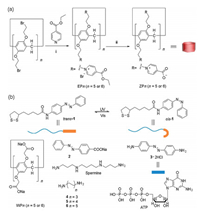
As shown in Fig. 1a, ZP5 and ZP6 were synthesized by substitution reaction of bromo-substituted PA[n]s, followed by hydrolysis of ester. ZPns are composed of equal numbers of carboxylic acid and pyridinium groups. Under neutral physiological condition, carboxylic acid groups are deprotonated, and ZP5 and ZP6 possess equal amount of cationic and anionic groups. By contrast, at acidic pH, carboxylic acid groups are protonated to render the host molecule cationic. For comparison purpose, carboxylated PA[n]s (WP5-WP6) were prepared (Fig. 1b). Azobenzene-based neutral guest 1 was synthesized, and conjugated to GNP surface by gold-thiol bond. Azobenzene is known to undergo reversible conversion between trans- and cis-configurations under UV or visible irradiation [37]. GNPs were prepared by citric acid-catalyzed reduction reaction of HAuCl4. Subsequently, guest 1 was conjugated to GNPs. Transmission electron microscopy (TEM) showed that GNPs were relatively uniform with an average size of 16 nm (Fig. S32 in Supporting information). Zeta-potential indicated that guest 1-conjugated GNPs (bare GNPs) were negatively charged (−29 mV).ZP5 and ZP6 were incubated with GNPs to conjugate zwitterionic host molecules by host-guest interaction (Fig. 2a). ZP6 coating enhanced ζ-potential to −22 mV (Fig. S34 in Supporting information). PEG- and WP5/WP6- coated GNPs were prepared for comparison purpose. Similar GNP morphology was observed by TEM (Fig. S32). While WP6-coated GNPs were highly negatively charged (−26 mV), PEG-coated GNPs were slightly negatively charged (−7 mV).
|
Download:
|
| Fig. 1. (a) Synthetic procedure for ZP5-ZP6. ⅰ) EtOH, 90 ℃, 48 h. ⅱ) NaOH, H2O, reflux, 24 h. (b) Chemical structures of carboxylated PA[n]s WP5, WP6, guests 1-6, and biomolecules spermine and ATP. | |

|
Download:
|
| Fig. 2. (a) TEM images of ZP6-GNPs. (b) Hydrodynamic diameter (nm) of bare-GNPs, WP6-GNPs, ZP6-GNPs and PEG-GNPs after incubation in FBS (50%) for 30 h. | |
First, we investigated host-guest chemistry in sodium phosphate buffer. Neutral guest 1 was poorly soluble in water. We used diamino azobeneze (guest 3, positively charged) and carboxylated azobenzene (guest 2, negatively charged) as model guests. ZP5-ZP6 and WP5-WP6 were used as host molecules. Neutral pH (7.4) and acidic pH (5.5) were used to study the influence of solvent acidity. Binding constant (Ka) value was determined by indicator displacement assay with rhodamine 6 G or acridine orange as the dye. As shown in Table 1, ZP5-ZP6 could tightly bind guests 2–3 with similar binding affinity. Upfield shift of azobenzene proton resonances in 1H NMR spectra showed that trans-azobenzene were encapsulated by ZPn cavity (Fig. S31 in Supporting information). By contrast, cis-isomer of azobenzene could not be encapsulated by ZPn based on 1H NMR spectroscopy. It was discovered that acidic pH had limited impact toward Ka value. Therefore, ZP5-ZP6 were high affinity host molecules for trans-isomers of azobenzene guests at neutral or acidic pH. Similarly, WP5-WP6 could bind guest 3 with high affinity (Figs. S29 and S30 in Supporting information).
|
|
Table 1 The value of Ka (L/mol) for the complex of ZP5-ZP6 and guests 2-6 or biomolecules spermine/ATP at neutral (pH 7.4) or acidic (pH 5.5) condition. |
Model cationic guests 4–6, and biomolecules spermine and adenosine triphosphate (ATP) were chosen to further explore host-guest chemistry. ZP5-ZP6 showed selective binding toward guests 4–6 with different lengths. ZP5-ZP6 demonstrated significantly lower binding affinity toward positively-charged biomolecule spermine and negatively-charged biomolecule ATP, compared to azobenzene-guests 2–3. The selective binding may ensure stable supramolecular encapsulation under complex physiological condition.
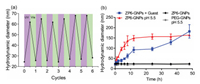
Second, stability of nanoparticles was evaluated in blood serum. Fetal bovine serum (FBS) was used to mimic blood serum condition. Bare or coated GNPs were incubated in 50% FBS at 37 ℃, and aliquots of nanoparticle suspension were extracted to separate GNPs from solvent by centrifugation. Supernatant was removed and GNPs were re-suspended in ultrapure water to determine nanoparticle size by dynamic light scattering (DLS). As shown in Figs. 2b and 3a, after 30 h incubation, hydrodynamic size of bare GNPs significantly increased in FBS.WP5/WP6-coated GNPs also had significant enhancement in size. These three groups of GNPs were lack of nonfouling-coating layer to protect them from nonspecific adsorption of serum proteins and other biomolecules in FBS, which resulted in aggregation and nanoparticle size growth. By sharp contrast, ZP5/ZP6-coated GNPs had a constant hydrodynamic size of 24 nm for up to 48 h. The "stealth" effect of ZP5-ZP6 coating was superior compared to that of PEG: PEG-coated GNPs showed an increase in hydrodynamic size after 10 h, while ZPn-coated GNPs maintained their size. Consequently, ZPns were excellent nonfouling coating material for GNPs.

|
Download:
|
| Fig. 3. (a) Zavg hydrodynamic diameter (nm) of bare-GNPs, WP5-GNPs, WP6-GNPs, ZP5-GNPs, ZP6-GNPs and PEG-GNPs in FBS (50%). (b) Zavg hydrodynamic diameter (nm) of ZP6-GNPs in FBS (50%), after being washed with FBS for three times. | |
Host-guest interaction is non-covalent, and could be unstable in blood serum, especially when exposed to competitive biomolecular guests. To determine the stability of surface coating, ZP6-coated GNPs were incubated in FBS for 0.5 h, and isolated from the solution by centrifugation. After repeated for three times, GNPs were re-suspended in fresh FBS (50%), and size distribution was monitored by DLS. As shown in Fig. 3b, nanoparticle size maintained at 24 nm for up to 7 d, which demonstrated that ZP6-coating layer was retained even after extensive washing by FBS. We attributed the stability to high affinity binding between ZPn and guest 1. Although FBS is composed of complex biomolecular species, minimal competitive displacement occurred. We expect ZPn-coated GNPs to be stable during blood circulation.
Next, we studied the stimuli-responsiveness of ZPn nonfouling coating layer. ZP5/ZP6-coated GNPs were incubated in 10% FBS, and UV-irradiation (365 nm) was applied for 2 h. Hydrodynamic size of GNPs in water was monitored by DLS. As shown in Fig. 4a, hydrodynamic size of nanoparticles significantly increased. We believe the reason was that guest 1 transformed to cis-isomer, and ZPn coating layer was removed from GNP surface, which led to serum protein adsorption and GNP aggregation. Subsequently, GNP suspension was irradiated with visible light for 2 h to allow cis-azobenzene to convert to trans-isomer. GNPs regained smaller hydrodynamic size in ultrapure water, which showed that ZPn-nonfouling coating layer attached to nanoparticle surface via host-guest interaction. This reversible process could be repeated for up to six cycles.
|
Download:
|
| Fig. 4. (a) Zavg hydrodynamic diameter (nm) of ZP6-GNPs in FBS (10%) treated with UV/visible irradiation. (b) Zavg hydrodynamic diameter (nm) of ZP6-GNPs with or without guest 3, ZP6-GNPs at pH 5.5, PEG-GNPs at pH 5.5; condition: FBS (10%). Temperature: 37 ℃. Error bar was calculated based on triplicated measurements. | |
Similarly, ZPn-coated GNPs were responsive to competitive displacement and acidic pH. As shown in Fig. 4b, guest 3 was used as competitive guest, and added to a suspension of ZP6-coated GNPs in 10% FBS. It was discovered that the addition of competitive guest 3 would result in the enhancement in GNP hydrodynamic size. To study responsiveness to acidic pH, ZP6- or PEG-coated GNPs were incubated in 10% FBS at pH 7.0 or pH 5.5. As shown in Fig. 4b, at pH 5.5, ZP6-coated GNPs increased in hydrodynamic size, while PEG-coated GNPs retained their nonfouling ability. Based on Ka value for the complex of ZP6 and guests 2–3 under neutral and acidic conditions, we believe ZP6 formed stable supramolecular complex with guest 1 on GNPs at acidic pH (Table 1). The reason for this pH-responsiveness was the protonation of carboxylic acid groups, and conversion from zwitterionic to cationic host molecule. As shown in Fig. S34, ζ-potential of ZP6-coated GNPs increased from −26 mV at pH 7.4 to −16 mV at pH 5.5. Similar observations had been reported for zwitterionic polymer-coated GNPs in literature [14].
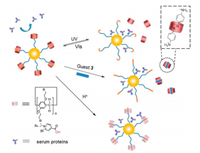
As summarized in Scheme 1, ZPn-coated GNPs were responsive to three stimuli types: photo-irradiation, competitive guest and acidic pH. Under stimuli, nonfouling and ultra-stable GNPs could expose inner core or undergo surface charge conversion, which may be used to enhance interaction between GNP and biological interface.
|
Download:
|
| Scheme 1. Cartoon depicting surface coating of GNPs with ZP6 via host-guest interaction to achieve nonfouling effect and multistimuli responsiveness. | |
Lastly, we investigated the influence of ZPn-based nonfouling coating layer toward cell uptake efficiency of GNPs. Nonfouling coating layer of ZPn could render nanoparticles "stealth" to protect them from immunological clearance during circulation in blood (Fig. 5b). When reaching target tissues, an enhanced cell uptake of GNPs was preferred to improve therapeutic efficacy or chromophore accumulation. Biocompatibility of ZP5-ZP6 was evaluated by using human normal cell line MRC-5, and host molecules were confirmed to be nontoxic (Fig. S35 in Supporting information). Human cancer HeLa cell line was used to determine cell uptake efficiency. HeLa cells were incubated with bare or coated GNPs, which were subsequently washed and lysed to determine GNP cell uptake by inductively coupled plasma atomic emission spectroscopy (ICP-AES). As shown in Fig. 5a, bare GNPs had significantly higher cell uptake efficiency compared to that of ZP6- or PEG-coated GNPs.

|
Download:
|
| Fig. 5. (a) Cell uptake of bare-GNPs, PEG-GNPs, ZP6-GNPs, ZP6-GNPs after UV and ZP6-GNPs with guest measured by ICP-AES. Each data point represents an average value ± standard deviation from three independent measurements. (b) Cartoon depicting stable ZP6-coated GNPs in blood serum, and UV- or guest-induced enhancement in cell uptake. | |
We studied the influence of stimuli-responsiveness toward cell uptake. ZP6-coated GNPs were treated with UV-irradiation or competitive guest 3 before incubation with cells, and GNP uptake was determined by ICP-AES. It was discovered that UV-irradiation and competitive displacement resulted in an enhancement of cell uptake by 5.9- and 7.4-fold, respectively. Acid-induced cell uptake enhancement of zwitterionic polymer-coated GNPs was reported in literature [14]. Consequently, external stimuli could lead to the conversion of surface characteristics to improve uptake efficiency by tumor cells. Surface property was reported to be critical for GNP endocytosis pathways and cell uptake efficiency [38]. We believe the removal of ZP6-nonfouling coating could expose GNP-core, and enhance interaction with cell membrane receptors or even alter internalization pathway. By using host-guest interaction, nanoparticles were ultra-stable in blood serum due to nonfouling effect of zwitterionic host molecules, and efficiently internalized by tumor cells after applying stimuli.
In conclusion, we report a new multistimuli-responsive nonfouling coating strategy for nanoparticles with zwitterionic PA[n]s. By coating with ZPn, nanoparticles were ultra-stable in blood serum. UV-irradiation, competitive displacement or acidic pH could be used to switch off ZPn-nonfouling effect, by which enhancement of cell uptake may be achieved. This strategy may be explored for targeted-delivery of therapeutic or diagnostic agents in vivo.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentThe authors are grateful to National Natural Science Foundation of China (Nos. 21672042 and 21921003) for financial support.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.08.001.
| [1] |
W. Fan, B. Yung, P. Huang, X. Chen, Chem. Rev. 117 (2017) 13566-13638. DOI:10.1021/acs.chemrev.7b00258 |
| [2] |
G. Chen, I. Roy, C. Yang, P.N. Prasad, Chem. Rev. 116 (2016) 2826-2885. DOI:10.1021/acs.chemrev.5b00148 |
| [3] |
A.E. Nel, L. Mädler, D. Velegol, et al., Nat. Mater. 8 (2009) 543-557. DOI:10.1038/nmat2442 |
| [4] |
I. Banerjee, R.C. Pangule, R.S. Kane, Adv. Mater. 23 (2011) 690-718. DOI:10.1002/adma.201001215 |
| [5] |
M.B. Flanagan, M. Lundqvist, J. Stigler, T. Cedervall, T. Bergga, ACS Nano 9 (2011) 7503-7509. |
| [6] |
B.A. Hucknall, S. Rangarajan, A. Chilkoti, Adv. Mater. 21 (2009) 2441-2446. DOI:10.1002/adma.200900383 |
| [7] |
H. Otsuka, Y. Nagasaki, K. Kataoka, Adv. Drug Deliv. Rev. 64 (2012) 246-255. DOI:10.1016/j.addr.2012.09.022 |
| [8] |
J. Chen, J. Ding, Y. Wang, et al., Adv. Mater. 29 (2017) 1701170. DOI:10.1002/adma.201701170 |
| [9] |
E. Ostuni, R.G. Chapman, R.E. Holmlin, S. Takayama, G.M. Whitesides, Langmuir 17 (2001) 5605-5620. DOI:10.1021/la010384m |
| [10] |
P. Zhang, F. Sun, S. Liu, S. Jiang, J. Control. Release 244 (2016) 184-193. DOI:10.1016/j.jconrel.2016.06.040 |
| [11] |
S. Jiang, Z. Cao, Adv. Mater. 22 (2010) 920-932. DOI:10.1002/adma.200901407 |
| [12] |
W. Yang, L. Zhang, S. Wang, A.D. White, S. Jiang, Biomaterials 30 (2009) 5617-5621. DOI:10.1016/j.biomaterials.2009.06.036 |
| [13] |
L. Zhang, Z. Cao, T. Bai, et al., Nat. Biotechnol. 31 (2013) 553-556. DOI:10.1038/nbt.2580 |
| [14] |
T. Mizuhara, K. Saha, D.F. Moyano, et al., Angew. Chem. Int. Ed. 54 (2015) 6567-6570. DOI:10.1002/anie.201411615 |
| [15] |
Y. Yuan, C. Mao, X. Du, et al., Adv. Mater. 24 (2012) 5476-5480. DOI:10.1002/adma.201202296 |
| [16] |
J.Z. Du, T.M. Sun, W.J. Song, J. Wu, J. Wang, Angew. Chem. Int. Ed. 49 (2010) 3621-3626. DOI:10.1002/anie.200907210 |
| [17] |
J.M. Zayed, N. Nouvel, U. Rauwald, O.A. Scherman, Chem. Soc. Rev. 39 (2010) 2806-2816. DOI:10.1039/b922348g |
| [18] |
D.S. Kim, J.L. Sessler, Chem. Soc. Rev. 44 (2015) 532-546. DOI:10.1039/C4CS00157E |
| [19] |
T. Ogoshi, T. Yamagishi, Y. Nakamoto, Chem. Rev. 116 (2016) 7937-8002. DOI:10.1021/acs.chemrev.5b00765 |
| [20] |
S.J. Barrow, S. Kasera, M.J. Rowland, J. Del Barrio, O.A. Scherman, Chem. Rev. 115 (2015) 12320-12406. DOI:10.1021/acs.chemrev.5b00341 |
| [21] |
X. Ma, Y. Zhao, Chem. Rev. 115 (2015) 7794-7839. DOI:10.1021/cr500392w |
| [22] |
G. Yu, K. Jie, F. Huang, Chem. Rev. 115 (2015) 7240-7303. DOI:10.1021/cr5005315 |
| [23] |
D. Mao, Y. Liang, Y. Liu, et al., Angew. Chem. Int. Ed. 56 (2017) 12614-12618. DOI:10.1002/anie.201707164 |
| [24] |
Y. Chen, S. Sun, D. Lu, Y. Shi, Y. Yao, Chin. Chem. Lett. 30 (2019) 37-43. DOI:10.1016/j.cclet.2018.10.022 |
| [25] |
T. Xiao, L. Zhou, L. Xu, et al., Chin. Chem. Lett. 30 (2019) 271-276. DOI:10.1016/j.cclet.2018.05.039 |
| [26] |
L.L. Zhao, Y. Han, C.G. Yan, Chin. Chem. Lett. 31 (2019) 81-83. |
| [27] |
L. Gao, M. Li, S. Ehrmann, Z. Tu, R. Haag, Angew. Chem. Int. Ed. 58 (2019) 3645-3649. DOI:10.1002/anie.201810314 |
| [28] |
L. Jiang, X. Huang, D. Chen, et al., Angew. Chem. Int. Ed. 56 (2017) 2655-2659. DOI:10.1002/anie.201611973 |
| [29] |
Y. Chang, K. Yang, P. Wei, et al., Angew. Chem. Int. Ed. 53 (2014) 13126-13130. DOI:10.1002/anie.201407272 |
| [30] |
D. Cao, Y. Kou, J. Liang, et al., Angew. Chem. Int. Ed. 48 (2009) 9721-9723. DOI:10.1002/anie.200904765 |
| [31] |
Y. Cao, X. Hu, Y. Li, et al., J. Am. Chem. Soc. 136 (2014) 10762-70769. DOI:10.1021/ja505344t |
| [32] |
S. Li, H. Zhang, X. Xu, Y. Liu, Nat. Commun. 6 (2015) 7590. DOI:10.1038/ncomms8590 |
| [33] |
L. Chen, W. Si, L. Zhang, et al., J. Am. Chem. Soc. 135 (2013) 2152-2155. DOI:10.1021/ja312704e |
| [34] |
Z. Li, Y. Zhang, C. Zhang, et al., J. Am. Chem. Soc. 136 (2014) 8577-8589. DOI:10.1021/ja413047r |
| [35] |
L. Luo, G. Nie, D. Tian, et al., Angew. Chem. Int. Ed. 128 (2016) 12905-12908. DOI:10.1002/ange.201603906 |
| [36] |
H. Li, Y.W. Yang, Chin. Chem. Lett. 24 (2013) 545-552. DOI:10.1016/j.cclet.2013.04.014 |
| [37] |
D. Xia, G. Yu, J. Li, F. Huang, Chem. Commun. 50 (2014) 3606-3608. DOI:10.1039/c3cc49686d |
| [38] |
Y. Jiang, S. Huo, T. Mizuhara, et al., ACS Nano 10 (2015) 9986-9993. |
 2021, Vol. 32
2021, Vol. 32 


