b School of Pharmacy, Hunan University of Chinese Medicine, Changsha 410208, China
Along with the social development, cancer therapy is one of the biggest challenges worldwide [1]. Traditional cancer treatments such as surgical operating excision, chemotherapy and radiotherapy have some unavoidable disadvantages. For example, cancer metastasis may occur when tumor tissue has not been excised completely [2]. Multidrug resistance of tumors may emerge due to long term chemotherapy, which makes it more difficult to cure cancer. Furthermore, healthy tissues or organs may be damaged due to repeated treatments, which may lead to serious side effects such as vomiting and fatigue [3]. Therefore, it is important to design multifunctional integrated therapeutic delivery nanoplatforms combined with different therapeutic form such as gene therapy [4], photodynamic therapy (PDT) and photothermal therapy (PTT) [5].
PTT has recently been known as a promising cancer treatment modality [6, 7], which involves injection of materials with high photothermal conversion efficiency into the patient, gathering around the tumor tissues by targeted or untargeted recognition. In the process of local heating, tumor cells undergo denaturation and cell membrane destruction, and cells suffer irreversible damage. Moreover, the temperature is closely related to the damage of DNA. When the amount of DNA damaged exceeds a certain percentage, cells are easy to undergo necrosis and apoptosis [8]. Thermal ablation typically causes the tumor cell necrosis, which may produce tumor related antigens. Consequently, the body's immune system can be stimulated and activated by antigens to kill tumor cells forcefully, thus controlling the development of primary and metastatic cancers. In addition, PTT can increase the blood perfusion of the tumor, hence affecting tumor microenvironment and reducing the hypoxic area [7].
However, PTT is often limited to the thermo-resistance of tumor cells mediated by the overexpression of HSPs [9]. Recent research showed that PTT could cause heat shock response, due to the sensitivity of tumor tissues to heat stimulation [10, 11]. The fundamental cellular defense mechanism of heat shock response may trigger the resistance of cancer cells and reduce the therapeutic efficacy [12], but HSP small interfering RNA (siRNA) could inhibit the expression of HSP, thus overcoming the disadvantages of PTT to some extent.
Since its discovery in 1998 [13], RNA interference (RNAi) using siRNA to treat a variety of diseases by blocking the production of target proteins has proven to be one of the most promising techniques [14]. Recent studies have shown that introduction of the single-stranded double stranded RNA (dsRNA) into the cell can cause photothermal and RNAi, leading to its corresponding gene silencing. These post-transcriptional gene silencing are called RNAi, which are small pieces of RNA synthesized artificially in vitro during RNAi. Recently, nanoparticles (NPs) are commonly used as non-viral gene carriers for siRNA in cancer treatment (Fig. 1). However, it still faces many challenges, especially the lack of effective siRNA delivery carriers [15, 16].
|
Download:
|
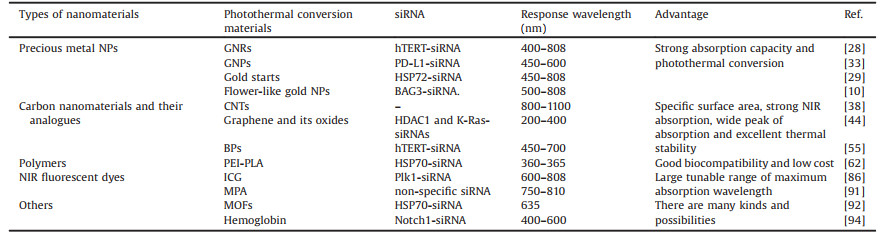
| Fig. 1. Schemes of representative siRNA delivery platforms that have been used in clinical studies. (A) Liposome, (B) DPCTM and (C) GalNAc siRNA conjugates. Reproduced with permission [15]. Copyright 2019, The Journal of Gene Medicine. | |
When co-treat with photothermal and RNAi, RNAi can play a role in gene silencing therapy, and PTT can play a role in phototherapy. At the same time, photothermal conversion material as a good delivery carrier contributes a lot to the delivery of siRNA, such as human telomerase reverse transcriptase (hTERT) siRNA. Therefore, it is important to combine PTT with gene therapy materials to achieve photothermal and RNAi synergistic therapy. The key component of photothermal and RNAi synergistic therapy is photothermal conversion material. Among a wide variety of stimuli-responsive platforms, near-infrared (NIR) light responsive nanocarriers have gained wide concerns as they can penetrate into deeper tissues, accurately control drug release, and exert low damage to normal tissues [17]. With the rapid development of nanotechnology, some nanomaterials with NIR response have been reported and applied in photothermal and RNAi synergistic therapy.
Therefore, in this review, we discuss a variety of photothermal conversion nanomaterials, such as gold NPs, carbon nanotubes and indocyanine green (ICG) [18], which have been used as PTT materials or siRNA delivery systems in photothermal and RNAi synergistic therapy [19], and become one of the research hotspots of nanomedicine. Preceding this discussion, we provide a brief summary of PTT and RNAi as background for the subsequent sections
2. Applications of photothermal conversion materials in photothermal and RNAi synergistic therapy 2.1. Precious metal nanomaterialsPrecious metal NPs used in PTT include but not limited to gold, silver [20], platinum [21, 22], palladium [23]. All these precious metal nanomaterials have a strong local surface plasmon resonance effect, which endows them with superior absorption capacity for NIR light, and the ability to convert energy from light into heat with effective photothermal conversion [24]. Due to the excellent photothermal conversion performance and good biological inertia property, precious metal materials have become the most thoroughly studied and widely used photothermal nano-conversion materials in the area of PTT [25, 26]. However, their disadvantages are high cost, poor photothermal stability and certain toxicity. Therefore, some additional nanomaterials have been developed to overcome these issues [27].
Gold nanorods (GNRs) could inhibit the cancer cell collective migration by altering the actin filaments with the introduction of NIR light [28], and cell junctions with remarkable triggered phosphorylation changes of essential proteins. GNRs Taken together, PTT have promoted the development of light-mediated PTT for cancer treatment over the past few decades [27]. Most solid tumors with triple negative breast cancer (TNBC) as an example, can not distinguish themselves from normal cells due to the lost of recognizable surface markers. Therefore, it is a challenging task to selectively identify and block those damages by PTT [29]. A type of siRNA can silence the expression of HSP by HSPs or BAG3 genes as an effective carrier of RNA interference (RNAi), so HSP siRNA can inhibit heat shock response and facilitate cancer cells to be more sensitive to PTT. BAG3 gene is efficient to inhibit the expression of heat-shock response. Qin et al. designed a therapeutic system, which consisted of GNRs and hTERT siRNA assembled on the surface of ZnCa2O4: Cr nanofibers. This system can enhance the uptake of GNRs, and had the ability of carrying and subsequent release of siRNA in cytoplasm [28]. The GNRs with surface modification had the ability to deliver targeting BAG3 siRNA oligos [12]. When the synthesized nanocomplex delivered siRNA into cancer cells, they exhibited unexceptionable ability in the delivery of siRNA into cancer cells and high silencing efficiency, which was superior over than the commercial Lipofectamine 2000 [30]. The combination of GNRs with siRNA has substantial potential in clinical cancer therapy. Gene silencing can deal with the therapeutic resistance of PTT and enhance their photothermal therapeutic efficacy. Both activities simultaneously amplify the gene silencing effect of siRNA, suggesting that the combination of GNRs with siRNA represents an efficient therapeutic platform.
Besides GNRs, there are many other structures of gold NPs that have been widely studied [31]. For instance, polyethylene glycol (PEG) 5000 coated nanoshells with diameter about 150 nm have been approved by the US Food and Drug Administration (FDA). At present, gold nanoshells can be used for the treatment of neck, head, and metastatic lung cancer in clinical trials [32]. Gold nanoprisms (GNPs) and gold nanostar have also been broadly studied for the potential applications in both imaging and tumor treatment on account of their special characteristics [33]. PTT could selectively sensitize the TNBC, thus enhancing the accuracy of cure by gold nanostar/siHSP72/hyaluronic acid (HA) targeting CD44 (surface molecule of TNBC-overexpressed) and reducing HSP72 subsequently [29]. siRNA could be delivered by the gold to tumor cells that overexpress integrin. The survival rate of tumor cells under NIR laser irradiation was significantly lower than that of PTT or gene therapy alone [34].
In another study, a safe and biodegradable flower-like gold NPs platform for synergistic gene silencing and photothermal therapy was prepared by layer-by-layer strategy based on anhydride NPs synthesized from soft template cetyltrimethylammonium chloride [10]. Yan et al. constructed the gold nanocages/polyethylenimine (PEI)/microRNA (miRNA)/HA complex also by layer-by-layer strategy. It can effectively delivery miRNA to target cells and significantly enhance its antitumor effect through a combination of gene therapy and PTT, and may be developed for the treatment of liver cancer [35].
2.2. Carbon nanomaterials and their analoguesCarbon-based NPs are characterized by large specific surface area, strong NIR absorption, wide peak of absorption and excellent thermal stability [36]. Photothermal conversion agents collaborative siRNA delivery fevers first research using the mesoporous carbon NPs with great loading efficiency and effective protection of siRNA through pore space [37]. Carbon nanotubes (CNTs) and graphene are two of the most widely studied carbon-based nanomaterials [38], CNTs are so-called graphene, which is a cylinder with a diameter of several hundred nanometers and a length of several microns. It was thought to be part of the graphene crystal structure until 2004 [39], when the graphene layer was mechanically stripped off and separated from the sheet of graphene crystal, showing amazing electrical properties [40, 41]. Including large ratio of surface area to volume and excellent electrical and optical properties, it has better photoacoustic (PA) and photothermal effects [42]. A CD44 targeted photoacoustic nanophototherapy drug, ICG single-wall CNTs on the HA NPs, which was shrouded in coupled to theranostic nanometer complex forming. The tumor temperature raised to above 55 ℃ due to the photothermal effect of ICG and single-walled CNTs, which remarkably inhibited the growth of tumor and induced tumor cell death without apparent systemic and local toxic effects found in the mice [43]. It has also been documented that graphene oxide (GO) nanosheets have similar therapeutic effects [44-46].
Qian et al. synthesized high crystalline carbon nanodots with one high crystalline carbon nanocore and one hydrophilic surface. The NPs contained powerful light sound, electricity spectral analysis performance, style fluorescent radiation excellent water distribution, and effective NIR radiation, which could kill tumor cells without apparent damage to normal tissues [47]. The central neurological tumors such as glioma are currently a challenge to nanomaterial design. Specifically, these tumors build up in brain, which urgently promotes the fall of double mode image guide with antitumor properties while without obvious damage to normal tissues [47].
The great success of graphene in biological applications has accelerated the emergence of its analogues such as transition metal disulphide [48, 49] and black phosphorus (BP) [50, 51]. There is a higher expectation for these novel hybrid nanomaterials to co-treat tumors. Kong's synthesized dendrimer-modified MoS2 nanoflakes for combinational gene silencing and PTT. In their study, the xenografted 4T1 tumor model was treated with the combination of superpower therapy in vivo. The results proved that MoS2 nanoflakes represented a potential nanoplatform for combination therapy [52].
BPs is a kind of new nonmetal layer two-dimensional semiconductor materials, which has wide absorption spectroscopy from ultraviolet to NIR and good thermal performance [53]. Researchers have synthesized BPs with different thickness and size for biological imaging and treatment. It was important that the BP nanosheets could be gradually decomposed into non-toxic phosphate ions in vivo, such as phosphoric and phosphate [54].
So far, studies have suggested that negatively-charged siRNA is unable to adsorb the anionic BP nanosheets, so researchers used positively charged PEI to functionalize the BP nanosheets by simply mixing as-exfoliated at room temperature, stirring BP nanosheets with PEI in an ultrasonic bath [55]. With a simple step the enclosure directly coated the BPs nanosheet with tannins acid (TA)-Mn2+ chelate networks. The combined TA-Mn2+ a volumetric BPs nanosheets have the capacity to enhance T1 MRI contrast enhancement capability, outstanding optical imaging performance, and high photothermal conversion efficiency, thus holding great promise for imaging guides imaging PTT [56].
It has been reported that hTERT was closely related to tumor transformation, growth, and metastasis. Therefore, the design of siRNA to knockdown hTERT for tumor treatment represents a promising technique. In Chen's work [55], a biodegradable delivery system-small and thin BPs nanosheets was harnessed to deliver hTERT siRNA. Poly(ethylene glycol) (PEG) and PEI qualified BPs nanosheets were equipped with high siRNA loading capacity and strong cellular uptake. Thus, PPBP-siRNA is successful by the synergistic combination of PTT, targeted gene therapy and PDT.
Wang et al. designed another siRNA delivery system based on PEI functionalized BPs nanosheets, and the system significantly inhibited tumor cells growth [57]. Phosphorus is a necessary trace element, accounting for 1% of the body mass [58]. Results showed that BPs NPs and quantum dots were both easy to respond to oxygen and water, and the final degradation products are non-toxic phosphates [59, 60] that were not only the metabolite of human body, but also the substrate of other biological reactions in the body. Owing to the photothermal properties of BP nanosheet, this system had a good therapeutic effect on tumors, and would be a promising tool for clinical applications in the future. Adjusted to the photothermal properties of the BP nanosheets, the presented delivery system has a remarkable therapeutic effect on tumors.
2.3. PolymersInorganic nanomaterials are usually difficult to biodegrade and easy to store for a long time in the body, which may create potential long-term toxicity. Compared with inorganic molecules, polymers have advantages of good biocompatibility, degradability [61], and low synthesis cost. Therefore, polymer is a kind of PTT material with promising application prospect [62].
2.3.1. Ordinary polymersUnder NIR irradiation, the heat generated by polyaniline NPs can hinder the production of cellular components required for DNA and RNA synthesis, and thereby inhibited cell growth, proliferation, apoptosis and eventually death. Zhou et al. reported the synthesis of an agent for photoacoustic imaging-guided PTT of tumors, polyaniline-modified γ-polyglutamic acid nanogels. This nanoplatform may be loaded with other elements, which has multifunctions such as imaging, therapy and target, thus representing an excellent platform for multimode imaging and co-therapy of novel biosystems [63].
Dopamine is a neurotransmitter related to the regulation of mood in the body and hence has good biocompatibility [64]. Therefore, polydopamine can overcome the problem of poor biocompatibility of traditional PTT materials when being used for tumor PTT [11, 65]. Wang et al. constructed mesoporous dopamine NPs with size less than 100 nm and high photothermal conversion efficiency. By biomineralization successfully built on cationic NPs of CaP coating, in order to prevent the premature release. In addition, the tertiary amine and pyrocatechol on the surface of the carrier can provide simple and efficient solutions, based on obstacles of combination of photothermal conversion agents and gene therapy [66].
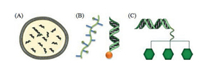
Zhang et al. prepared pH-responsive gene vector polydopamine-PEI-rPEG by modifying polydopamine NPs with low molecular weight PEI1.8k and PEG-phenylboric acid (PBA). PBA and polydopamine formed a pH-responsive borate bond. Due to PEG modification, the complex remained stable in the circulating pH environment or in extracellular delivery (pH 7.4). Boronate ester bonds can be cleaved after being internalized into endosomes. As shown in Fig. 2, the pH response of polydopamine-PEI-rPEG may promote complex dissociation and intracellular gene release. The good photothermal conversion ability of polydopamine makes the endosome escape quickly and overcomes many obstacles of gene transfer [67].
|
Download:
|
| Fig. 2. (A) Schematic representation of complexes preparation. (B) Possible mechanism of the PEG dissociation under acidic pH value. Reproduced with permission [67]. Copyright 2019, ACS Applied Materials & Interfaces. | |
Bioprobes prepared from polydopamine perfluorocarbon nanodroplets and biocompatible infrared-780 (IR-780) possess high photothermal conversion efficiency. These bioprobes have better imaging capabilities and can also be used as a PA contraption or photopyretic [68]. Polypyrrole NPs have strong optical absorption with NIR irradiation, which is widely used in biosensors, drug delivery and nerve regeneration [69]. There are still some polymers [70], which are not described in detail in this review.
2.3.2. Semiconductor polymersSemiconducting polymers are theranostic systems for NIR-II fluorescence imaging and PTT under safe laser fluence [71]. In fact, semiconductor polymers have been reported for PTT. For example, Lyu reported a biodegradable SPN with two optical active ingredients in its major semiconductor and optical polymer dopant fullerenes. Due to the densely populated in the NPs, vinyl key increased the mass absorption coefficient, thus improving the photoacoustic effect and the thermal efficiency. Therefore, these SPN NPs had oxidation properties, and thus can be easily degraded in peroxidase [72].
2.3.3. Supramolecular polymersSupramolecular polymers have important and various applications in catalysis and photothermal materials science by self-assembled from metal complexes [73]. As a new entry of dynamic and non-covalent polymers, supramolecular polymers specific structural and physical chemistry of the changes show the specific structure. At the same time, it can change the structure reversible, shape and function under various external excitement. In the future there are many academic researches applying to industrial widespread [74]. There are several unique advantages of functional supramolecular polymers, including the degradation capability of polymer backbones, smart responsiveness of biological stimuli, and easy biofunctionalization with bioactivity and targeting moieties, thereby showing remarkable reserve force for a wide range of biomedical applications.
The properties of supramolecular polymers containing metal can be altered by introducing metal ions with different functions and distributors. In turn, Pd(Ⅱ), Cu(Ⅰ), Cu(Ⅱ), Co(Ⅱ), Pt(Ⅱ) and Au(Ⅰ) complexes can also be functionalized with intriguing physical and chemical properties by introduction of functional polymeric nanostructures [75, 76].
As separate organic conjugated polymer contrast is low and the background of tumor intake is poor, the application of inorganic nanomaterials for cancer diagnosis and treatment is limited. In this context, the fabrication of organic-inorganic hybrid nano materials can make use of their advantages [69]. In the one side, inorganic materials have excellent optical properties, which can improve the thermal stability and photothermal conversion efficiency of the hybrid materials [77]. Furthermore, pharmaceutical organic polymers are capable of reducing the cure concentration of inorganic materials and the corresponding residual amount retained in the body [78].
2.4. NIR fluorescent dyesOrganic small molecular fluorescent dyes with NIR absorption, such as ICG and BODIPY [79], have been widely used for photoacoustic imaging and fluorescence imaging. ICG is an NIR fluorescence dye for a wide range of biological applications, and it is characterized by remarkable molar extinction coefficient and fluorescence quantum yield, low melting point and tunable range maximum absorption wavelength. Zheng et al. proved a potential application foreground for tumor diagnosis and target image formation due to high water stability, excellent NIR optical properties and remarkable internal targeting property of ICG. However, restrictions on the stability of extractive water, concentration-dependent aggregation, rapid infusion of the body, and lack of target specificity [80].
ICG has been approved by the US FDA for clinical NIR fluorescence imaging reagents. ICG is also a kind of good photosensitizer which can quickly absorb light and transfer heat to deep tissues after irradiation by specific wavelength light. Therefore, ICG has good application prospect in the field of tumor imaging diagnosis and treatment [81]. However, the application of ICG in diagnosis and treatment of diseases is limited by its instability and poor optical stability. It has been found that ICG loading into nanomaterials can improve drug stability, bioavailability and enhance targeted drug delivery [82]. Manganese dioxide (MnO2) NPs have the characteristics of good biocompatibility and strong adsorption, and can be degraded by glutathione overexpressed in cancer cells [83, 84]. ICG/MnO2 nanometer complex was prepared by loading ICG onto manganese dioxide nanometer sheet. The photothermal conversion efficiency of ICG/MnO2 solution gradually increases with the increase in solution concentration and illumination time in a certain range [85]. siRNA encapsulated with photosensitizer ICG to promote endosomal escape and conjugated with the siRGD peptide on the surface to penetrate tumor deeper [86]. ICG can be loaded into the internal cavity of polypeptide, which is rich in guanidine and has spherical helical polypeptide with remarkable multivalency-assisted membrane penetrating capability. siRNA against pyruvate kinase M2 (PKM2) form a positive nanocomplex. The silence of PKM2 can inhibit tumor glycolysis metabolism and further reduce the energy supply for HSPs production, hence hitting off the heat endurance of tumor cells, which consequently enhanced ICG-mediated photothermal ablation. Furthermore, energy consumption by PKM2 siRNA will lead to hunger of tumor cells and bring them to a halt [9, 87].
ICG-Der-02 (MPA), a derivative of ICG, possesses the capacity of both fluorescence emission and photothermal conversion under NIR laser irradiation [88, 89]. MPA could swiftly convert the absorbed NIR light energy into heat energy, thus quickly raising local temperature and killing tumor with NIR laser irradiating [90]. Multiple drug resistance can simultaneously hinder drug delivery and monitoring the delivery of chemotherapeutic agents. Therefore, Rui developed a new type of microcapsule system, which was loaded with siRNA and DOX by electrostatic absorption. MPA as a photothermal agent make drug delivery system successfully combined of the chemotherapy and photothermal therapy and control the silence of siRNA sequence, thus having a board potential for tumor combined therapy [91].
2.5. OthersPorous metal-organic frameworks (MOFs) nanostructures composed of metal ion/ion clusters and organic bridging ligands, and have the potential for biomedical applications. A novel zirconium-ferriporphyrin (Zr-FeP) MOF nanoshuttle is capable of generating abundant reactive oxygen species, thus having great photothermal effect with PTT conversion efficiency [92].
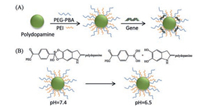
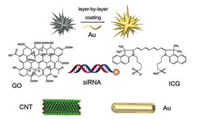
Hemoglobin is a natural photothermal molecule with remarkable biocompatibility and low immunogenicity [93], which can be used in PTT. In the meanwhile, functional selenium NPs could lead endogenous NO to endothelial cells, inhibiting the inflammatory response [94]. And then, they constructed PTT-triggered NO nanogenerators after loading Notch1-siRNA, the NPs could achieve a synergistic therapy with PTT, siRNA and NO, by lowering the level of inflammatory factors and macrophages and suppressing the inflammatory response. There are many photothermal conversion materials could be combined with siRNA (Table 1). Some common materials are displayed in Fig. 3.
|
|
Table 1 Common photothermal conversion materials combined with RNAi. |
|
Download:
|
| Fig. 3. Common photothermal conversion materials combined with gene therapy. | |
In the recent studies, we note that photothermal and RNAi synergistic therapy is often combined with other therapeutic techniques to assist in achieving better therapeutic effects [95]. In the following section, we will give a summary of some commonly used combinations.
Co-treatment by using NIR-induced photothermal and RNAi synergistic therapy with conventional drug molecules or biologics can create multimodal drug delivery systems [38, 96]. Additionally, the co-treat has been used to achieve synergistic antitumor effects recently [27]. And it is usually used in combination with vesicles to facilitate the fusion of cell membranes, which allowing antitumor drugs to enter tumor cells easily [97].
Phototherapy is a cure method to ablate tumor and inhibit growth through PDT and PTT triggered by light [43]. Cao decorated Cu2-xS on the MnO2 nanosheet surface, and loaded with HSP70 siRNA into the new nanosystem [98]. As a strong PA and photothermal imaging agent, the nanosystem had an advantage in the effectiveness of PTT/PDT due to siRNA-mediated blocking of heat-shock response and degrading of MnO2-related relieved tumors under a single NIR laser irradiation [99]. This nanomaterial can overcome the unavoidable defects, emphasizing their great promise in breaking the defense mechanism of tumor microenvironment. Due to the co-treatment effect of PDT and low-temperature PTT, with NIR laser irradiation, MOF nanoshuttles loaded with HSP70 siRNA could efficiently inhibit tumor growth both in vivo and in vitro [92].
Photoacoustic tomography (PAT) is an imaging technique that converts photo to acoustic signals based on adsorption photothermal energy, and have been demonstrated to have substantial potential for the biomedical application [100, 101]. It have been found that a variety of photothermal conversion materials could be used as efficient contrast agents for PAT, such as GNPs [34], black phosphorus and CuS NPs [56, 102], which could be used to delivery siRNA. After intravenous injection, co-treatment of tumor by using the hybrid materials under the PA/CT imaging can accurately position and thoroughly eliminate tumor in vivo [103].
4. ConclusionAlthough photothermal and RNAi synergistic therapy is going well, photothermal conversion material is always the key component of it. Photothermal conversion materials with enhanced permeability and retention effect can be targeted easier to tumor tissue. It could accumulate more to tumor tissue, and less in normal tissues and organs, thus significantly reducing the systemic toxicity improving antitumor efficiency.
Nevertheless, there are still some major challenges for photothermal and RNAi synergistic therapy. Thus it is often combined with antitumor drugs, such as DOX and epirubicin, for therapeutic applications. It can also be combined with vesicle compounds to facilitate the fusion of cell membranes. Besides, there are some other forms of combinations, such as PDT/imaging. The combination of various treatment techniques improved the therapeutic effect against tumor. But when using photothermal conversion material for cancer chemotherapy, the long-term toxicity is a major problem, especially for non-biodegradable inorganic NP carriers. Therefore, developing novel nanocarriers with good biocompatibility is an urgent task for clinical application
In addition, the biggest obstacle of photothermal conversion material is the depth of light penetration. Therefore, it is critical to develop corresponding medical devices to permit the permeation of laser to deeper tissues [104]. On the other hand, the real-time imaging monitoring of nanocarriers is of great significance [105]. The low stability of NIR fluorescent dye limits their application. Thus we need to find a suitable irradiation time [17] and optimize the structures and surface properties of drugs, so as to improve their antitumor efficacy. The current means have shown excellent curative effect in animal experiments.
In summary, although photothermal and RNAi synergistic therapy is currently limited for clinical application, it is believable that their applications have broad prospects in imaging and antitumor therapy and so on.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the Hunan Provincial Natural Science Foundation of China (No. 2018JJ1019) and the Hu-Xiang Young Talent Program (No. 2018RS3094), and the National Natural Science Foundation of China (No. 31871003), the Beijing Institute of Technology Research Fund Program for Young Scholars and the Fundamental Research Funds for the Central Universities (No. 2018CX01023), the Young Elite Scientist Sponsorship Program of Beijing Association for Science and Technology.
| [1] |
F. Madia, A. Worth, M. Whelan, R. Corvi, Environ. Int. 128 (2019) 417-429. DOI:10.1016/j.envint.2019.04.067 |
| [2] |
S.F. Brockmoeller, N.P. West, World J. Gastroenterol. 25 (2019) 2887-2897. DOI:10.3748/wjg.v25.i23.2887 |
| [3] |
P. De Cicco, M.V. Catani, V. Gasperi, et al., Nutrients 11 (2019) 1514. DOI:10.3390/nu11071514 |
| [4] |
Y. Weng, Q. Huang, C. Li, et al., Mol. Ther. Nucleic Acids 19 (2020) 581-601. DOI:10.1016/j.omtn.2019.12.004 |
| [5] |
L. Kong, L. Xing, B. Zhou, L. Du, X. Shi, ACS Appl. Mater. Interfaces 9 (2017) 15995-16005. DOI:10.1021/acsami.7b03371 |
| [6] |
Z. Yang, Z. Sun, Y. Ren, et al., Mol. Med. Rep. 20 (2019) 5-15. |
| [7] |
B. Pelaz, C. Alexiou, R.A. Alvarez-Puebla, et al., ACS Nano 11 (2017) 2313-2381. DOI:10.1021/acsnano.6b06040 |
| [8] |
M. Stapf, U. Teichgraber, I. Hilger, Int. J. Nanomed. 12 (2017) 2793-2811. DOI:10.2147/IJN.S120969 |
| [9] |
J. Dang, H. Ye, Y. Li, et al., Biomaterials 223 (2019) 119463. DOI:10.1016/j.biomaterials.2019.119463 |
| [10] |
Y. Liu, M. Xu, Y. Zhao, et al., J. Mater. Chem. B 7 (2019) 586-597. |
| [11] |
Y. Liu, J. Xu, L. Liu, et al., J. Biomed. Nanotechnol. 15 (2019) 1771-1780. DOI:10.1166/jbn.2019.2806 |
| [12] |
B.K. Wang, X.F. Yu, J.H. Wang, et al., Biomaterials 78 (2016) 27-39. DOI:10.1016/j.biomaterials.2015.11.025 |
| [13] |
A. Fire, S. Xu, M.K. Montgomery, et al., Nature 391 (1998) 806-811. DOI:10.1038/35888 |
| [14] |
Y. Weng, H. Xiao, J. Zhang, X.J. Liang, Y. Huang, Biotechnol. Adv. 37 (2019) 801-825. DOI:10.1016/j.biotechadv.2019.04.012 |
| [15] |
B. Hu, Y. Weng, X.H. Xia, X.J. Liang, Y. Huang, J. Gene Med. 21 (2019) e3097. |
| [16] |
S. Yu, X. Bi, L. Yang, et al., J. Biomed. Nanotechnol. 15 (2019) 1135-1148. DOI:10.1166/jbn.2019.2751 |
| [17] |
A. Saneja, R. Kumar, D. Arora, et al., Drug Discov. Today 23 (2018) 1115-1125. DOI:10.1016/j.drudis.2018.02.005 |
| [18] |
C. Yang, J. Xu, D.D. Yang, et al., Chin. Chem. Lett. 29 (2018) 1421-1424. DOI:10.1016/j.cclet.2018.02.014 |
| [19] |
G.T. Gibney, L.M. Weiner, M.B. Atkins, Lancet Oncol. 17 (2016) e542-e551. DOI:10.1016/S1470-2045(16)30406-5 |
| [20] |
I. Grabowska-Jadach, D. Kalinowska, M. Drozd, M. Pietrzak, Biomed. Pharmacother. 111 (2019) 1147-1155. DOI:10.1016/j.biopha.2019.01.037 |
| [21] |
Y. Zhu, W. Li, X. Zhao, et al., J. Biomed. Nanotechnol. 13 (2017) 1457-1467. DOI:10.1166/jbn.2017.2446 |
| [22] |
C. Chang, C. Wang, C. Zhang, et al., J. Biomed. Nanotechnol. 15 (2019) 1744-1753. DOI:10.1166/jbn.2019.2803 |
| [23] |
A.L. de Andrade Querino, J.T. da Silva, J.T. Silva, et al., J. Biol. Inorg. Chem. 24 (2019) 1087-1103. DOI:10.1007/s00775-019-01719-5 |
| [24] |
S.Q. Yang, L.Z. Zhou, Y. Su, R. Zhang, C.M. Dong, Chin. Chem. Lett. 30 (2019) 187-191. DOI:10.1016/j.cclet.2018.02.015 |
| [25] |
W. Wei, X. Zhang, S. Zhang, G. Wei, Z. Su, Mater. Sci. Eng. C: Mater. Biol. Appl. 104 (2019) 109891. DOI:10.1016/j.msec.2019.109891 |
| [26] |
J. Wang, K. Ma, H. Wang, et al., J. Biomed. Nanotechnol. 15 (2019) 2164-2178. DOI:10.1166/jbn.2019.2843 |
| [27] |
Y. Wu, M.R.K. Ali, B. Dong, et al., ACS Nano 12 (2018) 9279-9290. DOI:10.1021/acsnano.8b04128 |
| [28] |
L. Qin, P. Yan, C. Xie, et al., Nanoscale 10 (2018) 13432-13442. DOI:10.1039/C8NR03802C |
| [29] |
S. Wang, Y. Tian, W. Tian, et al., ACS Nano 10 (2016) 8578-8590. DOI:10.1021/acsnano.6b03874 |
| [30] |
B. Hu, L. Zhong, Y. Weng, et al., Signal Transduct. Target. Ther. 5 (2020) 101. DOI:10.1038/s41392-020-0207-x |
| [31] |
X. Huang, Y.L. Yin, M. Wu, W. Zan, Q. Yang, Chin. Chem. Lett. 30 (2019) 1335-1340. DOI:10.1016/j.cclet.2019.02.019 |
| [32] |
S. Huo, S. Chen, N. Gong, et al., Bioconjug. Chem. 28 (2017) 239-243. DOI:10.1021/acs.bioconjchem.6b00488 |
| [33] |
B. Liu, W. Cao, G. Qiao, et al., Acta Biomater. 99 (2019) 307-319. DOI:10.1016/j.actbio.2019.08.046 |
| [34] |
P. Wei, J. Chen, Y. Hu, et al., Adv. Healthc. Mater. 5 (2016) 3203-3213. DOI:10.1002/adhm.201600923 |
| [35] |
L.J. Yan, X.H. Guo, W.P. Wang, et al., Curr. Cancer Drug Targets 19 (2019) 330-337. DOI:10.2174/1568009618666181016144855 |
| [36] |
A.D. Tjandra, J. Huang, Chin. Chem. Lett. 29 (2018) 734-746. DOI:10.1016/j.cclet.2018.03.017 |
| [37] |
X. Du, C.X. Zhao, M.Y. Zhou, et al., Small 13 (2017) 1602592. DOI:10.1002/smll.201602592 |
| [38] |
B.S. Wong, S.L. Yoong, A. Jagusiak, et al., Adv. Drug Deliv. Rev. 65 (2013) 1964-2015. DOI:10.1016/j.addr.2013.08.005 |
| [39] |
K.R. Ratinac, W. Yang, S.P. Ringer, F. Braet, Environ. Sci. Technol. 44 (2010) 1167-1176. DOI:10.1021/es902659d |
| [40] |
K.S. Novoselov, A.K. Geim, S.V. Morozov, et al., Science 306 (2004) 666-669. DOI:10.1126/science.1102896 |
| [41] |
D. de Melo-Diogo, R. Lima-Sousa, C.G. Alves, I.J. Correia, Biomater. Sci. 7 (2019) 3534-3551. DOI:10.1039/C9BM00577C |
| [42] |
Z.M. Markovic, L.M. Harhaji-Trajkovic, B.M. Todorovic-Markovic, et al., Biomaterials 32 (2011) 1121-1129. DOI:10.1016/j.biomaterials.2010.10.030 |
| [43] |
G. Wang, F. Zhang, R. Tian, et al., ACS Appl. Mater. Interfaces 8 (2016) 5608-5617. DOI:10.1021/acsami.5b12400 |
| [44] |
F. Yin, K. Hu, Y. Chen, et al., Theranostics 7 (2017) 1133-1148. DOI:10.7150/thno.17841 |
| [45] |
D. Li, W. Nie, L. Chen, et al., J. Biomed. Nanotechnol. 14 (2018) 2003-2017. DOI:10.1166/jbn.2018.2646 |
| [46] |
X.M. Li, Y.H. Zhang, Z.Q. Ma, et al., Chin. Chem. Lett. 30 (2019) 489-493. DOI:10.1016/j.cclet.2018.03.019 |
| [47] |
M. Qian, Y. Du, S. Wang, et al., ACS Appl. Mater. Interfaces 10 (2018) 4031-4040. DOI:10.1021/acsami.7b19716 |
| [48] |
Z.H. Miao, L.X. Lv, K. Li, et al., Small 14 (2018) e1703789. DOI:10.1002/smll.201703789 |
| [49] |
M. Zhou, R. Zhang, M. Huang, et al., J. Am. Chem. Soc. 132 (2010) 15351-15358. DOI:10.1021/ja106855m |
| [50] |
Z. Guo, H. Zhang, S. Lu, et al., Adv. Funct. Mater. 25 (2015) 6996-7002. DOI:10.1002/adfm.201502902 |
| [51] |
J.R. Choi, K.W. Yong, J.Y. Choi, et al., Theranostics 8 (2018) 1005-1026. DOI:10.7150/thno.22573 |
| [52] |
L. Kong, L. Xing, B. Zhou, L. Du, X. Shi, ACS Appl. Mater. Interfaces 9 (2017) 15995-16005. DOI:10.1021/acsami.7b03371 |
| [53] |
L. Qin, S. Jiang, H. He, G. Ling, P. Zhang, J. Control. Release 318 (2020) 50-66. DOI:10.1016/j.jconrel.2019.12.013 |
| [54] |
P. Yasaei, B. Kumar, T. Foroozan, et al., Adv. Mater. 27 (2015) 1887-1892. DOI:10.1002/adma.201405150 |
| [55] |
L. Chen, C. Chen, W. Chen, et al., ACS Appl. Mater. Interfaces 10 (2018) 21137-21148. DOI:10.1021/acsami.8b04807 |
| [56] |
T. Guo, Y. Lin, G. Jin, et al., Chem. Commun. 55 (2019) 850-853. DOI:10.1039/C8CC08833K |
| [57] |
H. Wang, L. Zhong, Y. Liu, et al., Chem Commun. 54 (2018) 3142-3145. DOI:10.1039/C8CC00931G |
| [58] |
A.V. Skalny, W. Mona, R. Kao, et al., Biol. Trace Elem. Res. 191 (2019) 1-9. DOI:10.1007/s12011-018-1581-x |
| [59] |
X. Ling, H. Wang, S. Huang, F. Xia, M.S. Dresselhaus, Proc. Natl. Acad. Sci. U. S. A. 112 (2015) 4523-4530. DOI:10.1073/pnas.1416581112 |
| [60] |
H. Wang, X. Yu, Small 14 (2018) 1702830. DOI:10.1002/smll.201702830 |
| [61] |
S. Sharifi, S. Behzadi, S. Laurent, et al., Chem. Soc. Rev. 41 (2012) 2323-2343. DOI:10.1039/C1CS15188F |
| [62] |
L. Shao, Q. Li, C. Zhao, et al., Biomaterials 194 (2019) 105-116. DOI:10.1016/j.biomaterials.2018.12.021 |
| [63] |
Y. Zhou, Y. Hu, W. Sun, et al., Nanoscale 9 (2017) 12746-12754. DOI:10.1039/C7NR04241H |
| [64] |
H. Zhang, J. Wang, M. Hu, et al., Biomater. Sci. 7 (2019) 5177-5186. DOI:10.1039/C9BM01284B |
| [65] |
T. Sun, J.H. Dou, S. Liu, et al., ACS Appl. Mater. Interfaces 10 (2018) 7919-7926. DOI:10.1021/acsami.8b01458 |
| [66] |
Z.Q. Wang, L.C. Wang, N. Prabhakar, et al., Acta Biomater. 86 (2019) 416-428. DOI:10.1016/j.actbio.2019.01.002 |
| [67] |
P. Zhang, Q.N. Xu, X.F. Li, Y.X. Wang, Mater. Sci. Eng. C: Mater. 108 (2020) 11. |
| [68] |
H. Zhu, D. Qin, Y. Wu, et al., ACS Appl. Mater. Interfaces 10 (2018) 29251-29259. DOI:10.1021/acsami.8b08190 |
| [69] |
S. Wang, Z. Zhou, G. Yu, et al., ACS Appl. Mater. Interfaces 10 (2018) 28382-28389. DOI:10.1021/acsami.8b09670 |
| [70] |
M. Zheng, P. Zhao, Z. Luo, et al., ACS Appl. Mater. Interfaces 6 (2014) 6709-6716. DOI:10.1021/am5004393 |
| [71] |
Y. Yang, X. Fan, L. Li, et al., ACS Nano 14 (2020) 2509-2521. DOI:10.1021/acsnano.0c00043 |
| [72] |
Y. Lyu, Y. Fang, Q. Miao, et al., ACS Nano 10 (2016) 4472-4481. DOI:10.1021/acsnano.6b00168 |
| [73] |
Y. Dorca, E.E. Greciano, J.S. Valera, R. Gómez, L. Sánchez, Chemistry 25 (2019) 5848-5864. DOI:10.1002/chem.201805577 |
| [74] |
M.H. Bakker, C.C. Lee, E.W. Meijer, P.Y. Dankers, L. Albertazzi, ACS Nano 10 (2016) 1845-1852. DOI:10.1021/acsnano.5b05383 |
| [75] |
J.J. Zhang, W. Lu, R.W. Sun, C.M. Che, Angew Chem. Int. Ed. 51 (2012) 4882-4886. DOI:10.1002/anie.201108466 |
| [76] |
S. Wang, K.J. Chen, T.H. Wu, et al., Angew Chem. Int. Ed. 49 (2010) 3777-3781. DOI:10.1002/anie.201000062 |
| [77] |
J. Wang, X. Wu, P. Shen, et al., Int. J. Nanomedicine 15 (2020) 1903-1914. DOI:10.2147/IJN.S239751 |
| [78] |
X. Zheng, L. Wang, Y. Guan, et al., Biomaterials 235 (2020) 119792. DOI:10.1016/j.biomaterials.2020.119792 |
| [79] |
J.L. Donnelly, D. Offenbartl-Stiegert, J.M. Marín-Beloqui, et al., Chemistry 26 (2020) 863-872. DOI:10.1002/chem.201904164 |
| [80] |
C. Zheng, M. Zheng, P. Gong, et al., Biomaterials 33 (2012) 5603-5609. DOI:10.1016/j.biomaterials.2012.04.044 |
| [81] |
M. Wang, Y. Xiao, Y. Li, et al., Eur. J. Pharm. Sci. 134 (2019) 185-193. DOI:10.1016/j.ejps.2019.04.021 |
| [82] |
D. Hu, J. Zhang, G. Gao, et al., Theranostics 6 (2016) 1043-1052. DOI:10.7150/thno.14566 |
| [83] |
D. He, X. He, K. Wang, et al., Chem. Commun. 51 (2015) 776-779. DOI:10.1039/C4CC08172B |
| [84] |
Y.Z. Wang, Y.J. Song, G.X. Zhu, D.C. Zhang, X.W. Liu, Chin. Chem. Lett. 29 (2018) 1685-1688. DOI:10.1016/j.cclet.2017.12.004 |
| [85] |
R. Yang, M. Hou, Y. Gao, et al., Theranostics 9 (2019) 6314-6333. DOI:10.7150/thno.36252 |
| [86] |
Y. Wang, Y. Xie, K.V. Kilchrist, et al., ACS Appl. Mater. Interfaces 12 (2020) 4308-4322. DOI:10.1021/acsami.9b21214 |
| [87] |
H. Luo, Q. Wang, Y. Deng, et al., Adv. Funct. Mater. 27 (2017) 1702834. DOI:10.1002/adfm.201702834 |
| [88] |
Y. Hu, C. Chi, S. Wang, et al., Adv. Mater. 29 (2017) 1700448. DOI:10.1002/adma.201700448 |
| [89] |
X. Tan, S. Luo, L. Long, et al., Adv. Mater. 29 (2017) 1704196. DOI:10.1002/adma.201704196 |
| [90] |
Z. Sheng, D. Hu, M. Zheng, et al., ACS Nano 8 (2014) 12310-12322. DOI:10.1021/nn5062386 |
| [91] |
Y. Rui, B. Pang, J. Zhang, et al., Artif. Cells Nanomed. Biotechnol. 46 (2018) 15-24. |
| [92] |
K. Zhang, X. Meng, Y. Cao, et al., Adv. Funct. Mater. 28 (2018) 1804634. DOI:10.1002/adfm.201804634 |
| [93] |
J.X. Cheng, X.S. Xie, Science 350 (2015) aaa8870. DOI:10.1126/science.aaa8870 |
| [94] |
Y. Liu, L. Ma, H. Zhou, et al., J. Mater. Chem. B 6 (2018) 3497-3514. DOI:10.1039/C8TB00080H |
| [95] |
X. Tang, L. Tan, K. Shi, et al., Acta. Pharm. Sin. B 8 (2018) 587-601. DOI:10.1016/j.apsb.2018.05.011 |
| [96] |
Q. Guo, D. Wang, G. Yang, J. Biomed. Nanotechnol. 15 (2019) 2090-2099. DOI:10.1166/jbn.2019.2832 |
| [97] |
S. Khan, J. McCabe, K. Hill, P.A. Beales, J. Colloid Interface Sci. 562 (2020) 418-428. DOI:10.1016/j.jcis.2019.11.101 |
| [98] |
Y. Cao, X. Meng, D. Wang, et al., ACS Appl. Mater. Interfaces 10 (2018) 17732-17741. DOI:10.1021/acsami.8b05050 |
| [99] |
C. Ji, A. Yuan, L. Xu, et al., J. Biomed. Nanotechnol. 15 (2019) 311-318. DOI:10.1166/jbn.2019.2685 |
| [100] |
L.V. Wang, S. Hu, Science 335 (2012) 1458-1462. DOI:10.1126/science.1216210 |
| [101] |
Y. Li, D. Li, K. Jian, X. Mei, G. Wang, J. Biomed. Nanotechnol. 15 (2019) 85-99. DOI:10.1166/jbn.2019.2668 |
| [102] |
M. Zhou, G. Ku, L. Pageon, C. Li, Nanoscale 6 (2014) 15228-15235. DOI:10.1039/C4NR05386A |
| [103] |
J. Wang, X. Tan, X. Pang, et al., ACS Appl. Mater. Interfaces 8 (2016) 24331-24338. DOI:10.1021/acsami.6b08391 |
| [104] |
R.K. Nair, C. Christie, D. Ju, et al., Laser. Med. Sci. 33 (2018) 1747-1755. DOI:10.1007/s10103-018-2534-5 |
| [105] |
X. Meng, B. Zhang, Y. Yi, et al., Nano Lett. 20 (2020) 2522-2529. DOI:10.1021/acs.nanolett.9b05267 |
 2021, Vol. 32
2021, Vol. 32