The incidence and mortality of cancer have been increasing worldwide, making it one of the leading causes of death and a significant health burden in many countries [1]. Surgery, radiotherapy, chemotherapy, biotherapy and immunotherapy are several major approaches to treat this troublesome disease. Among these approaches, the latter three are basically relying on transferring some certain drugs or therapeutic agents into patients' blood circulation, which is not always well-tolerated by patients due to systematic toxicity or anaphylactic reactions when free drugs are directly administrated intravenously. A suitable drug delivering system might help to improve this situation by either enhancing the selectivity or providing a biocompatible shield outside the pharmaceuticals [2, 3].
In past decades, researchers have achieved numerous breakthroughs in molecular biology, pharmaceutics, and material science, which enables the invention of various novel drug delivery systems aiming to maximize the potency of the drug while minimizing the adverse effects. Most of these delivery systems were manufactured using polymers (e.g., polyethylene glycol (PEG)), liposomes, or nanoparticles [4]. Besides these artificial materials, some researchers focused on converting blood cells (erythrocytes, leukocytes, platelets, etc.) into natural carriers for intravenous drug delivery [5]. Among these natural carriers, erythrocyte-derived drug delivery system, also known as erythrocyte carriers, received most interest as a natural, biocompatible, biodegradable and easily accessible drug carrier.
Natural erythrocytes are non-nucleated biconcave disks. This special structure provides the cell a large surface-to-volume ratio and allows it to change in shape when passing through capillaries without rupturing its membrane. Erythrocytes have a life span of approximately 120 days. They express some immunomodulating molecules (e.g., CD47, DAF and CR1) on their surface, which might contribute to their long life-spans in the circulation [4, 6]. At the end of their life spans, erythrocytes are phagocytosed by the reticuloendothelial system (RES).
When erythrocytes are converted into drug carriers, these physiological properties might bring some remarkable and unique improvement to the therapeutic cargo. Comparing with free drugs, pharmaceuticals will retain a longer plasm half-life when coupling with erythrocytes [7]. In several specific cases, a slow, sustained drug release of pharmaceuticals has also been achieved, which is useful for most enzyme supplement therapies and delivery of targeted anti-cancer agents [8, 9].
The unique immunomodulating molecules expressing on the cell surface give erythrocyte carriers excellent biocompatibility, which offers us a brand new idea of "stealthy delivery" strategy. Protein drugs usually cause undesired allergic effects after repeated intravenous injections [10, 11], and their potency may also decrease due to the reaction with circulating antibodies or hydrolases [3]. However, after encapsulating them into erythrocytes, the immunogenicity can be effectively avoided and the drugs can also be protected from degradation by circulating hydrolases [3, 11]. For pharmaceuticals like nanoparticles, the erythrocyte coat can help them to evade the phagocytosis of RES and enable an ideal drug bio-distribution in vivo [12]. Besides protein drugs and nanoparticles, chemotherapeutic agents like daunorubicin [13] can also benefit from the "stealthy delivery" strategy to reduce systematic toxicity to normal tissue and improve patient's compliance.
Furthermore, as mature erythrocytes are restricted in blood vessels, liver, spleen and RES, erythrocyte-derived carriers are able to passively target cargoes to intravascular lesions or tissues with abundant blood supply. Based on this aspect of advantages, erythrocyte-derived carriers are well suited for delivering anti-leukemic drugs [13] and anticoagulant agents such as low molecular weight heparin (LMWH) [14] and plasminogen activators (PAs) [15]. For tissues with abundant blood supply, especially some hyper-vascular malignant neoplasms [16, 17], the erythrocyte-derived drug carrier is also a competitive candidate for delivering different kinds of anti-tumor agents.
Based on these advantages mentioned above, erythrocyte carriers remained one of the most reliable candidates for delivering various anti-cancer agents in treating malignancies. Current laboratory experiments have demonstrated many possibilities of this system by combining erythrocytes with different pharmaceuticals [18, 19], some of products have already begun clinical trials or been applied in routine clinical practice, and they all showed satisfying outcomes [20, 21].
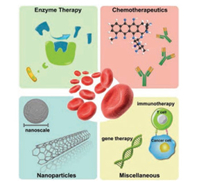
In this article, we will focus on revealing the wide application and enormous potential of erythrocyte-derived drug delivery systems in cancer therapy from an application point of view. We will review the development and recent progress about erythrocyte-derived drug delivery systems in cancer therapy from four different aspects of application: cancer enzyme therapy, delivering chemotherapeutic agents, combining with nanoparticles, and several miscellaneous anti-cancer agents (Fig. 1). In each aspect, some remarkable and iconic products will be illustrated. For each category of products mentioned, strategies to fabricate these products will be briefly discussed first, followed by elaborating the characteristics and advantages of each. Finally, a concise summary on this topic will be given, with some commentaries and an outlook for the future.
|
Download:
|
| Fig. 1. Four major aspects of the application of erythrocyte derived drug delivery system: cancer enzyme therapy (top-left), delivering chemotherapeutics (top-right), erythrocyte-camouflaged nanoparticles (bottom-left), and miscellaneous applications like cancer immune therapy and gene therapy (bottom-right). | |
In 1973, G. M. Ihler et al. reported the first research on using erythrocytes as drug carriers [9]. Their team successfully loaded β-glucosidase and β-galactosidase into erythrocytes to treat Gaucher's disease. Later on, other enzymes like adenosine deaminase and glutamine synthetase were also encapsulated in erythrocytes to treat different diseases like adenosine deaminase deficiency and hepatic encephalopathy [22, 23].
Just like the first research on erythrocyte-derived drug delivery system, the very first attempt to apply this novel drug delivery system in cancer therapy was likewise an enzyme therapy in which L-asparaginase (L-ASNase) was used to treat acute lymphoblastic leukemia (ALL) in 1960s [24]. So far, although iterative process has been made to improve the fabrication method of this enzyme-erythrocyte system, L-ASNase still remains the only enzyme which is used to treat malignancies.
The anti-cancer effect of L-asparaginase is based on the fact that certain cancer tissues cannot synthesize their own L-asparagine (L-ASN) and have to use circulating L-ASN to maintain their normal physiological function (protein synthesis, DNA replication, etc.) while normal human tissue can synthesize their own. Therefore, systematic depletion of circulating L-ASN by L-ASNase can impair the cell functions in certain cancer tissues, further leading to cell apoptosis and tumor regression [25, 26]. Besides ALL, low expression of asparagine synthetase (AS) was also found in pancreatic ductal adenocarcinoma (PDAC) [27], hepatocellular carcinoma (HCC) [28] and ovarian cancer [29]. Especially in PDAC, researchers discovered that pancreatic cancer cells were sensitive to L-ASNase both in vitro [27] and in vivo [30]. Clinic trials using erythrocyte-encapsulated L-ASNase (ASNase-ERY) with chemotherapy to treat PDAC showed prolonged overall survival (OS) and progression-free survival (PFS) [20, 31].
2.1. The fabrication of ASNase-ERYThere were several methods for encapsulating L-ASNase into erythrocytes, among which hypotonic treatment was one of the most common methods for this purpose. The concept of hypotonic treatment was still the basic concept for modern fabrication methods and several current commercial products manufactured by companies like EryDel [32, 33] or ERYTech [21].
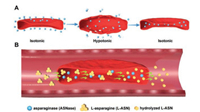
In this method, erythrocytes were first incubated with L-ASNase in a hypotonic medium. The hypotonic stress could create pores on the cell membrane of erythrocytes, then L-ASNase could pass through the pores and entered the inside of erythrocytes. Afterwards, the medium was recovered to isotonic solution, thus the pores on the membrane were closed and L-ASNase could be permanently entrapped into erythrocytes (Fig. 2A) [30]. After hypotonic treatment, L-ASNase was encapsulated within the erythrocyte and could circulate in the blood with it. As human erythrocyte membrane was permeable to L-ASN [34], this system could continuously pump circulating L-ASN into the erythrocyte and broke it down with the L-ASNase inside, which leaded to systematic depletion of L-ASN in plasma. In this system, erythrocytes acted more than a drug carrier, but also a micro-bioreactor for the hydrolytic reaction and a biocompatible shield for L-ASNase (Fig. 2B).
|
Download:
|
| Fig. 2. The fabrication and the application of erythrocyte-encapsulated asparaginase (ASNase-ERY). (A) Fabrication: Erythrocytes and ASNase were incubated together, then the medium was then turned into hypotonic. The hypotonic stress creates pores on the erythrocyte membrane, allowing ASNase to get to the inside of erythrocytes. After the medium was recovered to isotonic status, previous pores were closed and ASNase were entrapped in erythrocytes. (B) Application: ASNase-ERY acted as a micro-bioreactor in circulation. Circulating ASN was continuously pumped into ASNase-ERY and was hydrolyzed by ASNase inside. In the meanwhile, ASNase inside were also protected by the erythrocyte from being degraded in circulation. | |
There was always a concern that the membrane integrity of erythrocytes might be harmed by the hypotonic treatment, and further compromise the biocompatibility of this drug delivery system [35]. Solutions to this problem included several new approaches as double-dialysis procedure or other modified methods [36, 37]. Recently, protein-mediated encapsulation came to be another promising solution on this issue and showed better biocompatibility than hypotonic treatment [38, 39].
2.2. Advantages and applications of ASNase-ERYIn animal studies and human clinical trials, ASNase-ERY showed solid advantages in nearly every aspect when compared with free L-ASNase.
Free L-ASNase usually underwent premature degradation and inactivation by protease and RES in circulation, thus it had a relatively short plasma half-life [38]. ASNase-ERY, however, presented a much longer half-life which almost close to the nature half-life of erythrocytes [8, 40]. It also showed better pharmacodynamical parameters and efficiency in an animal experiment: circulating L-ASN was suppressed by 20 days when comparing with 10 days in the control group using free L-ASNase [41]. Another important improvement come with ASNase-ERY was immunocompatibility. Because of the bacterial origin of free L-ASNase used in clinical practice [42], allergic reaction was an inevitable problem. In contrast, there was no significant allergic effect observed in monkeys treated with ASNase-ERY [41]. Clinical trials in human ALL patients also showed ASNase-ERY group had less anaphylactic reactions than free L-ASNase group [11, 21]. Beside anaphylactic reactions, ASNase-ERY was also able to reduce the incidence of other adverse effects like coagulopathy, pancreatitis, and hepatic disorders caused by free L-ASNase [11, 21, 43]. Based on these advantages mentioned above, several researchers reported that, in animal models, ASNase-ERY would allow a 10-fold greater dose of L-ASNase than before without causing any noticeable anaphylaxis [44], and it could be administrated intraperitoneally as well [45]. In addition to ASNase-ERY, other attempts were tried to overcome the drawbacks of free L-ASNase as well, such as albumin [46], PEG [47] and other biomaterials [39]. Nevertheless, none of them could completely avoid the toxicity and the anaphylactic reaction as ASNase-ERY, and they might deteriorate, rather than improve, the therapeutic efficacy of L-ASNase [2, 39].
Several clinical trials about ASNase-ERY were conducted in the last decade, and most of them were focused on ALL. Clinical trials on GRASPA® (a type of asparaginase-encapsulated erythrocyte provided by ERYTech-France, Lyon, France) for ALL has entered phase II and III and confirmed the safety and the efficiency of this novel therapeutic agent, and elderly patients could also well tolerate with it [11, 21, 48]. Other studies included acute myelocytic leukemia (AML) (NCT01810705) and pancreatic cancer [20, 31, 49]. Especially in pancreatic cancer, latest published results on a phase IIb trial demonstrated that ASNase-ERY prolonged OS and PFS, and further phase III study was underway [31].
2.3. SummarizationIn summary, using erythrocyte-derived drug delivery systems for enzyme therapy in cancer is an effective and reliable method. Enormous progress has been made and promoted the translation of this technique into clinical practice in treating patients with ALL, AML, or PDAC. Future efforts might focus on trying this therapeutic agent on other solid malignant tumors expressing low AS like HHC and ovarian cancer.
3. Erythrocyte carriers in delivering chemotherapeutic agentsChemotherapy is still a crucial method in treating nearly every type of malignancies, most of cancer patients have to receive chemotherapy during their entire course of comprehensive anti-cancer therapy. However, a plenty of chemotherapeutic agents are cytotoxic drugs, they usually bring some undesired adverse and toxic effects to the patient when directly administrated intravenously. Besides, several chemotherapeutic agents may have a rather poor biocompatibility or affinity with tumor tissue, which will attenuate their therapeutic efficiency. Hence, there is always a need to reduce the toxicity of these chemotherapeutic agents and improve their biocompatibility. Now the erythrocyte-derived drug carrier seems to be a potential solution to this issue.
So far researchers have invented different erythrocyte carriers for delivering various kinds of chemotherapeutic agents, including not only traditional cytotoxic drugs (daunorubicin, cytosine arabinoside, etc.) [17, 50, 51] but also several novel anti-cancer monoclonal antibodies like rituximab [52]. All these erythrocyte carriers invented can be generally classified into three different categories, which represent three major eras in the development of erythrocyte carriers for chemotherapeutic agents: the whole erythrocyte, the red blood cell vesicles, the combination of nanomaterials and erythrocytes.
Because of the natural biodistribution of erythrocytes, the majority of chemotherapeutic agents delivered by erythrocyte carriers were likewise specific to treat hematopoietic malignancies. Among these agents, anthracycline antibiotics, covering daunorubicin (daunomycin) and doxorubicin (adriamycin), were the most well-studied ones. Therefore, this kind of agents will be used as an example to illustrate different kinds of erythrocyte carriers for delivering chemotherapeutic agents in the following paragraph.
3.1. Using the whole erythrocyte as a vehicleUsing the whole erythrocyte as a container or a vehicle, like ASNase-ERY, was the very first idea when coming to develop carriers for chemotherapeutic agents. Because hypotonic treatment was a relatively mature and simple procedure, it remained the dominant method for drug loading in early studies: chemotherapeutic drugs were first incubated with erythrocytes in the same medium. Then after the "hypotonic-isotonic" change of the medium, these drugs actively inflowed into the inside of erythrocytes during the "hypotonic phase" and were entrapped inside erythrocytes at the "isotonic phase". After being loaded with chemotherapeutic agents like daunomycin and adriamycin, erythrocyte carriers could still maintain the normal morphology and physiological function of erythrocytes [51, 53].
Besides the simple procedure of hypotonic treatment, there were some additional methods that could further improve this erythrocyte-chemotherapeutics delivery system. Treating drug-loaded erythrocytes with glutaraldehyde could improve the half-life and the concentration of the drug in plasma [54], leading to a better therapeutic effect for loaded drugs in vivo [55]. Besides, glutaraldehyde treatment was also able to increase hepatic uptake of treated erythrocytes and might inhibit the metastatic lesion of L1210 leukemic cells in the liver [56]. To avoid the possible damage to the membrane integrity of erythrocytes caused by hypotonic treatment, some researchers found that amphotericin B could be used as a mediator to trap daunomycin into erythrocytes instead of using hypotonic treatment [57]. However, this method was not further studied due to the invention of other erythrocyte carriers in the coming era.
In vivo studies on experimental animal models revealed erythrocyte-carried daunomycin did can increase the survival of mice bearing leukemic cells [51], which was in consistent with the previously observed improvements in pharmacodynamics [51, 53, 54, 58]. Furthermore, when chemotherapeutic agents were circulating in the circulation with erythrocytes for a long time, it might be helpful to eliminate circulating tumor cells and prevent the formation of distant hepatic or pulmonary metastatic lesions. Meanwhile, glutaraldehyde treated carriers would redound to control existed liver metastasis as their hepatic uptake was enhanced by the treatment [54, 56].
Even if entrapping these chemotherapeutic agents into erythrocytes was a leap forward, there was still a fatal flaw in this drug delivery system. Chemotherapeutic agents usually had a much smaller molecular weight than enzymes. An intact erythrocyte was relatively large for such small agents, making them unable to suit each other perfectly. Previous studies noticed that, for most of the time, entrapped agents would rapidly diffuse out of erythrocytes (about 85% drug release within 1 h) [51, 57], which was attributed to the small size of anthracyclines. Albeit glutaraldehyde treatment could slightly delay drug efflux from the carrier [55], it was still impossible to reach a controlled, slow release of the drug, thus other carriers were introduced subsequently.
In addition to using the erythrocyte as a container, another attempt was conjugating daunorubicin to the surface of erythrocytes via glutaraldehyde instead of encapsulating these drugs [59]. R. C. Gaudreault et al. reported their results, this new form of erythrocyte carrier showed a more superior therapeutic effect both in vitro and in vivo when compared with free drugs [59]. However, the author did not compare this new carrier with encapsulated daunorubicin. This technique was finally omitted and abandoned due to the invention of red blood cell vesicles.
3.2. Red blood cell vesiclesYears later, there came a brand-new drug delivery system called red blood cell vesicle (RBV), also known as nanoerythrosomes (nEryt). RBVs were small vesicles with the size of liposomes made by the extrusion of erythrocyte membrane [60]. As the optimal condition and process variables varied from different pieces of experimental objects (mice, rats, human beings, etc.), here was a brief summary for the general process of fabricating RBVs. First, erythrocytes were emptied of hemoglobin using a technique mimicking hypotonic treatment to create so called "erythrocyte ghosts". Then erythrocyte ghosts were washed and eventually suspended in a phosphate buffer for further filtration. To finally create RBVs, the erythrocyte ghost suspension should undergo several times of consecutive extrusion through a filter with pore size of 1 mm or less under nitrogen pressure [60-64]. Chemotherapeutic could be either covalently linked to RBVs or encapsulated inside RBVs.
There were also several ways to further enhance the therapeutic efficacy of RBVs. Among all multifarious methods reported, using folic acid (FA) to modify the surface of RBVs for improving selectivity of RBVs carrying doxorubicin (Dox-RBV) was the most accepted approach [65]. Experimental results showed FA-Dox-RBV had the highest cytotoxic activity on leukemic cells and provided the longest survival time in vivo among FA-Dox-RBV, Dox-RBV and Dox.
When chemotherapeutic agents were carried by RBVs, their therapeutic effect was enhanced which could be partially attribute to the longer plasma half-life and the slower release of loaded drug. When daunorubicin was covalently linked to RBVs, the cytotoxicity to P388D1 cells was similar to free daunorubicin in vitro, but in vivo studies demonstrated extend survival time than free drug control [60]. In the meanwhile, encapsulating anthracyclines into RBVs was evaluated by another team. It also presented enhanced half-life and increased bioavailability, which undoubtedly led to a better therapeutic effect [64].
Apart from the advantages in therapeutic aspects, RBVs might also be useful for attenuating the adverse effects brought by chemotherapeutic agents. For RBVs-encapsulated doxorubicin, researchers observed there was a less accumulation of doxorubicin in the heart on the administration of encapsulated doxorubicin than the free form [64]. This result suggested that the accumulative cardiac toxicity of doxorubicin, which was a great concern for clinical oncologists in their daily practice, could be reduced when the drug was carried by RBVs.
3.3. The combination of nanomaterials and erythrocytesWith the emergence of nanotechnology, the combination of nanomaterials and erythrocytes could give a great upgrade to previous erythrocyte carriers for delivering chemotherapeutic agents. In 2015, Qiang Fu et al. first developed an approach to co-deliver doxorubicin and paclitaxel in an erythrocyte membrane camouflaged nano-carrier for combination chemotherapy [66]. To create this novel carrier, they first successfully loaded three different agents (Fe3O4, paclitaxel and doxorubicin) on O-carboxymethyl-chitosan nanoparticles simultaneously. Then membrane of erythrocytes was covered on their nanoparticle by extruding the mixture of nanoparticles and erythrocytes through a polycarbonate porous membrane for 10 times.
Their product was proved being able to treat C57BL/6 mice bearing Lewis lung carcinoma cells effectively. In fact, their product showed the best result when compared with any other control products, suggesting using the combination of nanoparticles and erythrocytes might be a wise choice for delivering various kinds of chemotherapeutic agents [66].
Besides the melioration mentioned above, different unique characteristics brought by different nanomaterials could further reduce the adverse effect and improve the efficiency of chemotherapeutic agents. For example, Lang Rao et al. choosed to use nanoparticle with photocatalytic activity and erythrocyte membrane to encapsulate doxorubicin [67]. In their design, nanoparticles could let erythrocyte membrane degrade under ultraviolet irradiation, then doxorubicin could be precisely released at the site of tumor under the guidance of ultraviolet irradiation. Therefore, this novel design could enhance drug release in tumor lesion, preserve the biocompatibility brought by erythrocyte membrane in the circulation, and reduce undesired systematic toxicity in circulation. More details about nanoparticles and erythrocytes will be discussed in the next part of this review.
3.4. SummarizationAccording to the studies reviewed above, RBVs and camouflaged nanomaterials seemed superior to native erythrocytes, and they might be the future erythrocyte-derived carriers for chemotherapeutic agents.
Although studies about erythrocyte carriers and chemotherapeutic agents started just several years later than the first batch of studies on ASNase-ERY, now there is a clear gap between these two subjects. Only few studies about chemotherapeutic agents entered human clinical trials and was confined to native erythrocytes as carriers only [13]. No products derived from RBVs or nanomaterials have ever been tested in pre-clinical trials so far. This might be attribute to that we are still in the stage of discovering a better recipe, and there is no well-accepted, optimized protocol for the potential best carrier. Further studies might continue on looking for the best carrier, and pre-clinical trials should be planned for suitable candidates.
4. Erythrocyte carriers combine with nanoparticlesWith the technology breakthrough in material science and the application of nanotechnology in the field of health care, the advantages of nanomedicine, especially nanoparticles-based pharmaceuticals have made several exciting achievements and brought many marvelous products to this field. The delicate structure of nanoparticles would provide a better pharmacological effect to existing pharmaceuticals, such as high drug loading dose, accurate control of releasing, and enhanced passive accumulation in target tissue [68-70]. Moreover, certain inorganic nanomaterials produced from gold, iron or other polymers also brought us some novel and promising approaches to treat cancer like photothermic therapy (PTT) and photodynamic therapy (PDT) [71].
Nevertheless, currently using nanomaterials to build drug carriers are facing two major hurdles before they can be taken into next step and applied widely. First, a longer residence time in circulation is always preferred for most drugs. But nanomaterial-based carriers, no matter being administrated intravenously or subcutaneously, tend to be captured by macrophages and deposit in the RES immediately after injection rather than stay in circulation, thus only a small proportion of drugs can successfully enter circulation and finally reach the target tissue [12, 72]. Another issue is the potential toxicity of different nanoparticles. As some of them are made up of inorganic non-biodegradable materials or even heavy metal (gold, titanium, and so forth) [71], accumulation of these ingredients in organisms may lead to toxic effects to healthy tissues, which should never be neglected before conducting in vivo studies.
To deal with these hurdles, functionalizing nanoparticles with PEG, often termed PEGylation, was once regarded as the gold standard for improving the biocompatibility of nanoparticles [68, 73]. Although PEGylation could reduce the unintentional uptake by RES, the immunogenicity and toxicity coming along with PEGylation has already been noticed [74, 75]. Besides, PEGylation still could not give us a satisfying result on improving the circulating half-life of nanoparticles [76, 77]. These limitations subsequently motivated the search for alternatives for PEG. In an innovative research, CD47, an immunomodulating peptide presenting on the surface of erythrocyte, was used to decorate nanoparticles [78]. This small peptide enabled nanoparticles to have a longer circulating half-life than undecorated ones. On the foundation of this study, researchers started to take inspiration from nature to develop different carriers for nanoparticles. Cell membranes, especially erythrocyte membranes, seemed to be a brilliant choice.
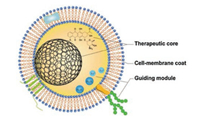
4.1. The fabrication of erythrocyte-camouflaged nanoparticlesIn recent years, erythrocyte-modified nanoparticles became a research hotspot in nanomedicine. There had not been many studies about cancer therapy until 2017, but the total number of researches on this topic experienced an incredible upsurge in last two years. Basic information for several important studies about erythrocyte-modified nanoparticles in cancer therapy was organized and listed in Table 1. A more detailed list of studies about erythrocyte-modified nanoparticles was listed in Table S1 (Supporting information). Preparing methods for those modified nanoparticles were not demonstrated in Table 1, which was because, to the extent of our knowledge, the combination of nanoparticles and erythrocytes in these studies shared the same pattern, and the product was termed as "camouflaged nanoparticles" (Fig. 3). Furthermore, nearly all the protocols for preparing different camouflaged nanoparticles were derived from the same archetypal procedure: the extrusion method, and it would be briefly described in the next paragraph.
|
|
Table 1 Summarization of several important studies about erythrocyte-membrane camouflaged nanoparticles in cancer therapy. |
|
Download:
|
| Fig. 3. The pattern and the concept of camouflaged nanoparticles. (1) Therapeutic core: Nanoparticles with or without other therapeutic agents (like chemotherapeutics); (2) Cell-membrane coat: derived from erythrocytes or/and other cells; (3) Guiding module: achieved by surface modification (targeting molecules) or internal components (like iron for magnetic-field guidance). Every component in each part can be substituted by any other compatible materials. | |
Summarizing from published works, there were three major steps in the extrusion method [72, 74]. First, erythrocytes should be washed to remove hemoglobin, and hypotonic treatment was recommended but not obligatory for removing some inner contents in the erythrocytes. Washed erythrocytes were named as erythrocyte ghosts. Secondary, erythrocyte ghosts were extruded serially through polycarbonate porous membrane with pores ranging of 100-400 nm (or membrane of other materials with pores in a similar size) to form erythrocyte-membrane-derived vesicles. Finally, nanoparticles and erythrocyte-membrane-derived vesicles were mixed together, and the mixture was further extruded through another porous membrane by several times. The machinal force imposed by extruding could facilitate fusing erythrocyte membrane onto the surface of nanoparticles, then camouflaged nanoparticles were fully prepared.
It is also easy to add some modificatory molecules on the surface of camouflaged nanoparticles to give them certain biological properties. Just by linking the molecule on the surface of native erythrocyte via different mediators before the second step, and these molecules would retain on the surface of camouflaged nanoparticles after several times of extrusion [75, 79, 80]. Recently some studies reported some improved methods for the "extrusion method", microfluidic electroporation-facilitated synthesis was one of them and showed promising results [81].
4.2. Advantages and applications of erythrocyte-camouflaged nanoparticlesOne of the most obvious advantage came with erythrocyte-camouflaged nanoparticles was that it could directly meliorate the biocompatibility of existing nanoparticles, they would stay longer in the circulation and exhibited a better therapeutic effect. This made it possible to deliver different nanoparticles efficiently regardless of their intrinsic physical characteristics.
Early experiments validated the superiority of camouflaged nanoparticles in improving drug efficacy and pharmacokinetic properties. Che-Ming J. Hu and colleagues first reported their work on erythrocyte membrane camouflaged poly(lactic-co-glycolic acid) (PLGA) nanoparticles in 2012 [74]. They revealed camouflaged nanoparticles could be effectively internalized by HeLa cells in vitro, and could retain in circulation for 72 h in vivo, which was much longer than the half-life of native nanoparticles. Later on, another study confirmed that doxorubicin delivered by camouflaged nanoparticles could exhibit higher cytotoxicity to cancer cells than free drugs and presented a longer plasma half-life than PEGylated ones [82]. After that, the in vivo safety and immunocompatibility of camouflaged nanoparticles was also confirmed in mice models [83]. Summarizing from these pilot studies, erythrocyte-camouflaged nanoparticles did better than PEGylation in preventing nanoparticles from being captured by RES and gave nanoparticles a better therapeutic effect. It might be a better substitution for PEGylation, which was previously considered gold standard for modifying nanoparticles.
The promising results from studies above gave researchers the confidence of administrating various nanoparticles in vivo without considering too much about their biocompatibility, which directly boosted the utility of novel therapeutic or diagnostic agents developed from nanomaterials.
PTT and PDT were two new nanomaterial-derived therapies for treating solid malignancies, but their application was hindered a lot because most of the PTT/PDT agents would deposit in RES and only a few of which would reach the lesion [72]. After being camouflaged with erythrocyte membrane, they deposited less in RES and presented even more promising anti-cancer activity in mice models [72, 80, 84-87]. In addition to PTT/PDT agents, camouflaged nanoparticles were also able to deliver nanovaccine (Hgp100) subcutaneously for induction of antitumor immunity against melanoma [88], and even siRNA for gene therapy of cancer [89, 90]. Beyond the application of treatment, camouflaged nanoparticles likewise contributed to cancer diagnosis by improve image quality via nano-contrast agents [80, 91], like camouflaged iron oxide nanoparticles (IONPs) [80]. Camouflaged nanoparticles would give the possibility of developing better contrast agents, which was previously consider non-biocompatible with organisms, for advanced imaging diagnosis.
Besides the melioration of biocompatibility, camouflaged nanoparticles were also capable of precise delivery of the drug or the therapeutic nanoparticle inside under biological or physiological guidance.
The phospholipid membrane on the surface of camouflaged nanoparticles could easily be modified with different molecules or peptides. Fang et al. reported their approach called "lipid-insertion", and camouflaged nanoparticles were successfully targeted to KB cells (nasopharyngeal cancer) or MCF-7 cells (breast cancer) after being modified with FA or AS1411 (a nucleolin-targeting aptamer) [79]. Besides small molecules, another team introduced a kind of hyaluronidase to the surface of camouflaged nanoparticles to hydrolyze hyaluronic acid (HA) in the extracellular matrix of cancer tissue [75]. This invention significantly enhanced the uptake of nanoparticles in PC3 cells which always showed resistance to therapeutics due to the protection of the abundant HA in the extracellular matrix [75, 92]. In another study, precise delivery was achieved by extracorporeal magnetic guidance with iron nanoparticles [66].
Generally, membrane-coated camouflaged nanoparticle could directly interact with target component in the cytoplasm after being internalized into cancer cells by membrane infusion [66]. For those nanoparticles which were designed to interact with the surface of the target cell, the membrane coat of camouflaged nanoparticles would help them avoid the clearance effect of RES in circulation, but it would hinder the interaction between nanoparticles and the surface of target cells in cancer tissue. But this dilemma was soon solved by another team, they successfully degrade the membrane coat of nanoparticle in cancer tissue by photocatalytic degradation [67]. This result gave us an encouraging message that, researchers now could let camouflaged nanoparticles to deliver the cargo to the inside or the outside of the cell according to their intents.
Despite all the advantages discussed above, camouflaged nanoparticles could only alter the administration and distribution of nanomaterials, but not the metabolism and elimination process. Nanomaterials would still deposit in organisms unless the material was naturally biodegradable, so the accumulative toxicity to human body was still a great concern to all the researchers and clinicians. Maybe in the future, there would be a method helping nanoparticles being excreted out of the organism, then it would definitely boost the translation of nanomedicine from laboratory to clinic.
Furthermore, to achieve the maximum efficacy of some nanoparticles, the membrane of erythrocytes sometimes would not always be the optimal choice. The membrane of other native cells (e.g., macrophages, platelets) or even cancer cells may be more suitable according to several published studies [68, 93-95], now there were some studies reported using combined membrane of erythrocyte and cancer cells to develop camouflaged nanoparticles [86, 96, 97].
4.3. SummarizationThere is another interesting fact: As we can conclude from currently available camouflaged nanoparticles, they all share a similar pattern (Fig. 3): a therapeutic core composed of nanoparticle with or without other therapeutic agents, a cell-membrane-derived coat on the surface of the therapeutic core, and an optional guiding module for targeting delivery which can be either attached on the surface (like FA) or encapsulated inside (like iron). According to the review above, each component in this pattern has many possible substitutes. Therefore, this gives us a formula for developing different camouflaged nanoparticles, we can choose the most suitable material in each component to develop different camouflaged nanoparticles with optimal biocompatibility and therapeutic efficacy to fulfill our different needs, as long as these materials are compatible with each other. There are still numerous formulas which have not been tested or even invented yet, so camouflaged nanoparticle is one of the most inspiring technologies in cancer therapy and has immeasurable potential in the future.
In the meantime, erythrocyte-camouflaged nanoparticles were facing a same problem as erythrocyte carriers for chemotherapeutic agents: the progress of clinical translation was relatively. The recent upsurge of studies on camouflaged nanoparticles brought us a variety of new formulations for camouflaged nanoparticles. Unfortunately, seldom studies tested the potential for clinical translation of these fancy nano-products. Maybe we should lay more emphasis on how to bring these camouflaged nanoparticles into clinical trials rather than being intent on developing new formulations of them.
5. Other application of erythrocyte carriers in cancer therapyBesides three major applications discussed above, erythrocyte carriers have also been introduced in some other aspects of cancer therapy.
Immunotherapy is an important part of the comprehensive management of cancer, and erythrocyte carriers have been proved being able to deliver various immunotherapy agents. Current studies showed that C-reactive protein associated with erythrocytes was able to trigger macrophage-mediated tumoricidal activity to lung metastasis of fibrosarcoma in mice [98]. Recombinant interlukin-2, a cytokine which could stimulate the proliferation and differentiation of lymphocytes into killer cells [99], could also be encapsulated into erythrocytes and perform their anti-tumor activity [100]. Apart from proteins discussed above, erythrocyte carriers could help to deliver cancer vaccines and enhance the efficacy of them as well. After being encapsulated in erythrocytes, these vaccines could successfully induce the antigen-specific T cell response and showed obvious inhibition of tumor growth, while unencapsulated free vaccines failed to do so [88, 101].
Recently, erythrocyte carriers were further used to deliver nucleic acids for cancer gene therapy. Huang et al. warped the minicircle DNA in the erythrocyte carriers and it could be successfully delivered to several human cancer cells. This result suggested the erythrocyte carrier could serve as a predominant gene delivery system and might promote the translation of cancer gene therapy into clinical practice [102].
6. ConclusionSince the very first study on erythrocyte-derived drug delivery system, plenty of progress have been made in the past four decades. As nature-derived vehicles, erythrocyte-derived delivering systems, also known as erythrocyte carriers, have some unique features, they are able to improve the pharmacokinetics, biocompatibility and biodistribution of drugs, enhance drug efficacy, facilitate targeting delivery of pharmaceuticals, and deliver large molecules or therapeutic particles stealthily without being recognized by immune system or being eliminated by RES. In view of these advantages, this promising delivering system has been deservedly introduced in cancer therapy. Current researches on erythrocyte carriers in cancer therapy showed inspiring results in four major aspects: cancer enzyme therapy, delivering chemotherapeutic agents, combining with nanoparticles, and several miscellaneous anti-cancer agents.
The application of erythrocyte carriers in cancer enzyme therapy was mainly about asparaginase. Now studies on this topic has entered clinical trials and returned satisfying results. For chemotherapeutic agents, several new erythrocyte carriers like RBVs were invented, and they were good at delivering drugs for hematopoietic malignancies. However, studies in this field were still restricted at pre-clinical stage. There were still some problems need to be overcome before it could be taken into the next stage. The combination of nanoparticles and erythrocytes gave birth of various different therapeutics for cancer, and they all showed encouraging results. As conclude above, the formulaic fabricating method for camouflaged nanoparticles could offer enormous possibilities to this delivering system, and now they have received much more attention over the world. Other miscellaneous anti-cancer agents, like agents for immunotherapy and gene therapy could also be efficiently delivered by erythrocyte carriers.
In summary, erythrocyte-derived drug delivery systems provide a great method for delivering different anti-cancer agents and they are capable of improving these agents in many aspects. This novel delivering system are now undergoing the translation process from laboratory to clinical practice, and might play an indispensable role in the management of cancer in the future.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 81472221). The authors thank the help from Prof. Yonghui Deng for revising the manuscript.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.08.048.
| [1] |
W. Chen, R. Zheng, P.D. Baade, et al., CA Cancer J. Clin. 66 (2016) 115-132. DOI:10.3322/caac.21338 |
| [2] |
F.M. Veronese, Biomaterials 22 (2001) 405-417. DOI:10.1016/S0142-9612(00)00193-9 |
| [3] |
M. Hamidi, A. Zarrin, M. Foroozesh, S. Mohammadi-Samani, J. Control. Release 118 (2007) 145-160. DOI:10.1016/j.jconrel.2006.06.032 |
| [4] |
C.H. Villa, D.B. Cines, D.L. Siegel, V. Muzykantov, Transfus. Med. Rev. 31 (2016) 26-35. |
| [5] |
S. Banskota, P. Yousefpour, A. Chilkoti, Macromol. Biosci. 17 (2017) 1600361. DOI:10.1002/mabi.201600361 |
| [6] |
P.A. Oldenborg, A. Zheleznyak, Y.F. Fang, et al., Science 288 (2000) 2051-2054. DOI:10.1126/science.288.5473.2051 |
| [7] |
K. Kinosita, T.Y. Tsong, Nature 272 (1978) 258-260. DOI:10.1038/272258a0 |
| [8] |
S.J. Updike, R.T. Wakamiya, E.N.J. Lightfoot, Science 193 (1976) 681-683. DOI:10.1126/science.821145 |
| [9] |
G.M. Ihler, R.H. Glew, F.W. Schnure, Proc. Natl. Acad. Sci. U. S. A. 70 (1973) 2663-2666. DOI:10.1073/pnas.70.9.2663 |
| [10] |
K. Adriaenssens, D. Karcher, A. Lowenthal, H.G. Terheggen, Clin. Chem. 22 (1976) 323-326. DOI:10.1093/clinchem/22.3.323 |
| [11] |
C. Domenech, X. Thomas, S. Chabaud, et al., Brit. J. Haematol. 153 (2011) 58-65. DOI:10.1111/j.1365-2141.2011.08588.x |
| [12] |
R.H. Fang, C.M.J. Hu, L. Zhang, Expert Opin. Biol. Th. 12 (2012) 385-389. DOI:10.1517/14712598.2012.661710 |
| [13] |
O.A. Skorokhod, T.T. Garmaeva, V.M. Vitvitsky, et al., Medical Sci. Monit. 10 (2004) I55-64. |
| [14] |
M. Müller, L. Büchi, K. Woodtli, A. Haeberli, J.H. Beer, FEBS Lett. 468 (2000) 115-119. DOI:10.1016/S0014-5793(00)01204-7 |
| [15] |
K. Ganguly, T. Krasik, S. Medinilla, et al., J. Pharmacol. Exp. Ther. 312 (2005) 1106-1113. DOI:10.1124/jpet.104.075770 |
| [16] |
M. Tonetti, E. Zocchi, L. Guida, et al., Adv. Exp. Med. Biol. 326 (1992) 307-317. |
| [17] |
U. Benatti, C. Polvani, L. Guida, E. Zocchi, A.D. Flora, Proc. Natl. Acad. Sci. U. S. A. 85 (1988) 3145-3149. DOI:10.1073/pnas.85.9.3145 |
| [18] |
C.H. Villa, A.C. Anselmo, S. Mitragotri, V. Muzykantov, Adv. Drug Deliver. Rev. 106 (2016) 88-103. DOI:10.1016/j.addr.2016.02.007 |
| [19] |
E. Briones, C.I. Colino, J.M. Lanao, J. Control. Release 125 (2008) 210-227. DOI:10.1016/j.jconrel.2007.10.027 |
| [20] |
J.B. Bachet, F. Gay, R. Maréchal, et al., Pancreas 44 (2015) 1141-1147. DOI:10.1097/MPA.0000000000000394 |
| [21] |
M. Hunault-Berger, T. Leguay, F. Huguet, et al., Am. J. Hematol. 90 (2015) 811-818. DOI:10.1002/ajh.24093 |
| [22] |
B.E. Bax, M.D. Bain, L.D. Fairbanks, et al., Eur. J. Haematol. 79 (2007) 338-348. DOI:10.1111/j.1600-0609.2007.00927.x |
| [23] |
E.A. Kosenko, N.I. Venediktova, A.A. Kudryavtsev, et al., Biochem. Cell Biol. 86 (2008) 469-476. DOI:10.1139/O08-134 |
| [24] |
J.M. Hill, J. Roberts, E. Loeb, et al., JAMA 202 (1967) 882-888. DOI:10.1001/jama.1967.03130220070012 |
| [25] |
F.F. Becker, J.D. Broome, Science 156 (1967) 1602-1603. DOI:10.1126/science.156.3782.1602 |
| [26] |
D.A. Cooney, R.E. Handschumacher, Annu. Rev. Pharmacolog. 10 (1970) 421-440. DOI:10.1146/annurev.pa.10.040170.002225 |
| [27] |
M.C. Wu, G.K. Arimura, A.A. Yunis, Int. J. Cancer 22 (1978) 728-733. DOI:10.1002/ijc.2910220615 |
| [28] |
B. Zhang, L.W. Dong, Y.X. Tan, et al., Br. J. Cancer 109 (2013) 14-23. DOI:10.1038/bjc.2013.293 |
| [29] |
P.L. Lorenzi, J.N. Weinstein, Drug News Perspect. 22 (2009) 61-64. DOI:10.1358/dnp.2009.22.1.1303819 |
| [30] |
Y. Godfrin, F. Horand, R. Franco, et al., Expert Opin. Biol. Th. 12 (2011) 127-133. |
| [31] |
P. Hammel, P. Fabienne, L. Mineur, et al., Eur. J. Cancer 124 (2020) 91-101. DOI:10.1016/j.ejca.2019.10.020 |
| [32] |
L. Chessa, V. Leuzzi, A. Plebani, et al., Orphanet J. Rare Dis. 9 (2014) 5. DOI:10.1186/1750-1172-9-5 |
| [33] |
V. Leuzzi, R. Micheli, D. D'Agnano, et al., Neurol. Neuroimmunol. Neuroinflamm. 2 (2015) e98. DOI:10.1212/NXI.0000000000000098 |
| [34] |
F.I. Ataullakhanov, V.M. Vitvitskiǐ, A.M. Zhabotinskiǐ, A.V. Pichugin, Biokhimiia 50 (1985) 1733-1737. |
| [35] |
C.M.J. Hu, R.H. Fang, L. Zhang, Adv. Healthc. Mater. 1 (2012) 537-547. DOI:10.1002/adhm.201200138 |
| [36] |
A. Naqi, J.R. DeLoach, K. Andrews, W. Satterfield, M. Keeling, Biotechnol. Appl. Biochem. 10 (1988) 365-372. |
| [37] |
R. Kravtzoff, C. Ropars, M. Laguerre, J.P. Muh, M. Chassaigne, J. Pharm. Pharmacol. 42 (1990) 473-476. |
| [38] |
Y.M. Kwon, H.S. Chung, C. Moon, et al., J. Control. Release 139 (2009) 182-189. DOI:10.1016/j.jconrel.2009.06.027 |
| [39] |
H. He, J. Ye, Y. Wang, et al., J. Control. Release 176 (2014) 123-132. DOI:10.1016/j.jconrel.2013.12.019 |
| [40] |
R. Kravtzoff, P.H. Colombat, I. Desbois, et al., Eur. J. Clin. Pharmacol. 51 (1996) 221-225. DOI:10.1007/s002280050187 |
| [41] |
S.J. Updike, R.T. Wakamiya, J. Lab. Clin. Med. 101 (1983) 679-691. |
| [42] |
M.H. Woo, L.J. Hak, M.C. Storm, et al., Leukemia 12 (1998) 1527-1533. DOI:10.1038/sj.leu.2401162 |
| [43] |
H.F. Oettgen, P.A. Stephenson, M.K. Schwartz, et al., Cancer 25 (1970) 253-278. DOI:10.1002/1097-0142(197002)25:2<253::AID-CNCR2820250204>3.0.CO;2-U |
| [44] |
S.J. Updike, Bibl. Haematol. 51 (1985) 65-74. |
| [45] |
M. Keeling, W. Satterfield, K. Andrews, J.R. DeLoach, Biotechnol. Appl. Biochem. 12 (1990) 331-335. |
| [46] |
M.J. Poznansky, M. Shandling, M.A. Salkie, J. Elliott, E. Lau, Cancer Res. 42 (1982) 1020-1025. |
| [47] |
V.I. Avramis, S. Sencer, A.P. Periclou, et al., Blood 99 (2002) 1986-1994. DOI:10.1182/blood.V99.6.1986 |
| [48] |
X. Thomas, C.L. Jeune, Int. J. Hematologic Oncol. 5 (2016) 11-25. DOI:10.2217/ijh-2016-0002 |
| [49] |
P. Hammel, J.B. Bachet, I. El-Hariry, et al., J. Clin. Oncol. 35 (2017) e15718. DOI:10.1200/JCO.2017.35.15_suppl.e15718 |
| [50] |
C. Barton, J.R. DeLoach, Am. J. Vet. Res. 43 (1982) 2210-2212. |
| [51] |
T. Kitao, K. Hattori, M. Takeshita, Experientia 34 (1978) 94-95. DOI:10.1007/BF01921924 |
| [52] |
R. Mukthavaram, G. Shi, S. Kesari, D. Simberg, J. Control. Release 183 (2014) 146-153. DOI:10.1016/j.jconrel.2014.03.038 |
| [53] |
A.D. Flora, U. Benatti, L. Guida, E. Zocchi, Proc. Natl. Acad. Sci. U. S. A. 83 (1986) 7029-7033. DOI:10.1073/pnas.83.18.7029 |
| [54] |
M. Tonetti, A.B. Astroff, W. Satterfield, et al., Am. J. Vet. Res. 52 (1991) 1630-1635. |
| [55] |
U. Benatti, E. Zocchi, M. Tonetti, et al., Pharmacol. Res. 21 (1989) 27-33. |
| [56] |
E. Zocchi, M. Tonetti, C. Polvani, et al., Proc. Natl. Acad. Sci. U. S. A. 86 (1989) 2040-2044. DOI:10.1073/pnas.86.6.2040 |
| [57] |
T. Kitao, K. Hattori, Cancer Res. 40 (1980) 1351-1353. |
| [58] |
A. Gasparini, M. Tonetti, M. Tonetti, et al., Adv. Exp. Med. Biol. 326 (1992) 299-304. |
| [59] |
R.C. Gaudreault, B. Bellemare, J. Lacroix, Anticancer Res. 9 (1989) 1201-1205. |
| [60] |
A. Lejeune, M. Moorjani, C. Gicquaud, et al., Anticancer Res. 14 (1994) 915-919. |
| [61] |
M. Moorjani, A. Lejeune, C. Gicquaud, et al., Anticancer Res. 16 (1996) 2831-2836. |
| [62] |
A. Lejeune, P. Poyet, R.C. Gaudreault, C. Gicquaud, Anticancer Res. 17 (1997) 3599-3603. |
| [63] |
J. Désilets, A. Lejeune, J. Mercer, C. Gicquaud, Anticancer Res. 21 (2001) 1741-1747. |
| [64] |
P.R. Mishra, N.K. Jain, Drug Deliv. 7 (2000) 155-159. DOI:10.1080/10717540050120197 |
| [65] |
P.R. Mishra, N.K. Jain, Drug Deliv. 10 (2003) 277-282. DOI:10.1080/drd_10_4_277 |
| [66] |
Q. Fu, P. Lv, Z. Chen, et al., Nanoscale 7 (2015) 4020-4030. DOI:10.1039/C4NR07027E |
| [67] |
L. Rao, Q.F. Meng, Q. Huang, et al., Adv. Healthc. Mater. 5 (2016) 1420-1427. DOI:10.1002/adhm.201600303 |
| [68] |
A. Kroll, R.H. Fang, L. Zhang, Bioconjugate Chem. 28 (2016) 23-32. |
| [69] |
O.C. Farokhzad, R. Langer, Adv. Drug Deliver. Rev. 58 (2006) 1456-1459. DOI:10.1016/j.addr.2006.09.011 |
| [70] |
K. Greish, J. Fang, T. Inutsuka, A. Nagamitsu, H. Maeda, Clin. Pharmacokinet. 42 (2003) 1089-1105. DOI:10.2165/00003088-200342130-00002 |
| [71] |
F. Yang, C. Jin, S. Subedi, et al., Cancer Treat. Rev. 38 (2012) 566-579. DOI:10.1016/j.ctrv.2012.02.003 |
| [72] |
J.G. Piao, L. Wang, F. Gao, et al., ACS Nano 8 (2014) 10414-10425. DOI:10.1021/nn503779d |
| [73] |
K. Knop, R. Hoogenboom, D. Fischer, U.S. Schubert, Angew. Chem. Int. Ed. 49 (2010) 6288-6308. DOI:10.1002/anie.200902672 |
| [74] |
C.M.J. Hu, L. Zhang, S. Aryal, et al., Proc. Natl. Acad. Sci. U. S. A. 108 (2011) 10980-10985. DOI:10.1073/pnas.1106634108 |
| [75] |
H. Zhou, Z. Fan, P.K. Lemons, H. Cheng, Theranostics 6 (2016) 1012-1022. DOI:10.7150/thno.15095 |
| [76] |
Y. Wang, Y. Liu, H. Luehmann, et al., ACS Nano 6 (2012) 5880-5888. DOI:10.1021/nn300464r |
| [77] |
Y. Wang, K.C.L. Black, H. Luehmann, et al., ACS Nano 7 (2013) 2068-2077. DOI:10.1021/nn304332s |
| [78] |
P.L. Rodriguez, T. Harada, D.A. Christian, et al., Science 339 (2013) 971-975. DOI:10.1126/science.1229568 |
| [79] |
R.H. Fang, C.M.J. Hu, K.N.H. Chen, et al., Nanoscale 5 (2013) 8884-8888. DOI:10.1039/c3nr03064d |
| [80] |
C. Wang, X. Sun, L. Cheng, et al., Adv. Mater. 26 (2014) 4794-4802. DOI:10.1002/adma.201400158 |
| [81] |
L. Rao, B. Cai, L.L. Bu, et al., ACS Nano 11 (2017) 3496-3505. DOI:10.1021/acsnano.7b00133 |
| [82] |
S. Aryal, C.M.J. Hu, R.H. Fang, et al., Nanomedicine 8 (2013) 1271-1280. DOI:10.2217/nnm.12.153 |
| [83] |
B.T. Luk, R.H. Fang, C.M.J. Hu, et al., Theranostics 6 (2016) 1004-1011. DOI:10.7150/thno.14471 |
| [84] |
H. Ding, Y. Lv, D. Ni, et al., Nanoscale 7 (2015) 9806-9815. DOI:10.1039/C5NR02470F |
| [85] |
D.M. Zhu, W. Xie, Y.S. Xiao, et al., Nanotechnology 29 (2018) 084002. DOI:10.1088/1361-6528/aa9ca1 |
| [86] |
D. Wang, H. Dong, M. Li, et al., ACS Nano 12 (2018) 5241-5252. DOI:10.1021/acsnano.7b08355 |
| [87] |
X. Liang, X. Ye, C. Wang, et al., J. Control. Release 296 (2019) 150-161. DOI:10.1016/j.jconrel.2019.01.027 |
| [88] |
Y. Guo, D. Wang, Q. Song, et al., ACS Nano 9 (2015) 6918-6933. DOI:10.1021/acsnano.5b01042 |
| [89] |
W. Ou, J.H. Byeon, Z.C. Soe, et al., Theranostics 9 (2019) 6780-6796. DOI:10.7150/thno.37123 |
| [90] |
T. Wang, Y. Luo, H. Lv, et al., ACS Appl. Mater. Inter. 11 (2019) 45455-45466. DOI:10.1021/acsami.9b16637 |
| [91] |
L. Rao, Q. Meng, L.L. Bu, et al., ACS Appl. Mater. Inter. 9 (2017) 2159-2168. DOI:10.1021/acsami.6b14450 |
| [92] |
N.C. Singha, T. Nekoroski, C. Zhao, et al., Mol. Cancer Ther. 14 (2015) 523-532. DOI:10.1158/1535-7163.MCT-14-0580 |
| [93] |
R.H. Fang, C.M.J. Hu, B.T. Luk, et al., Nano Lett. 14 (2014) 2181-2188. DOI:10.1021/nl500618u |
| [94] |
D. Dehaini, X. Wei, R.H. Fang, et al., Adv. Mater. 29 (2017) 1606209. DOI:10.1002/adma.201606209 |
| [95] |
Y. Liu, X. Wang, B. Ouyang, et al., J. Mater. Chem. B 6 (2018) 7033-7041. DOI:10.1039/C8TB02143K |
| [96] |
Y. Wang, Z. Luan, C. Zhao, C. Bai, K. Yang, Eur. J. Pharm. Sci. 142 (2020) 105136. DOI:10.1016/j.ejps.2019.105136 |
| [97] |
Q. Jiang, Y. Liu, R. Guo, et al., Biomaterials 192 (2019) 292-308. DOI:10.1016/j.biomaterials.2018.11.021 |
| [98] |
S. Gautam, B. Barna, T. Chiang, J. Pettay, S. Deodhar, J. Biol. Response Mod. 6 (1987) 346-354. |
| [99] |
S.A. Rosenberg, J. Immunol. 192 (2014) 5451-5458. DOI:10.4049/jimmunol.1490019 |
| [100] |
D.H. Mitchell, G.T. James, C.A. Kruse, Biotechnol. Appl. Biochem. 12 (1990) 264-275. |
| [101] |
A. Banz, M. Cremel, A. Mouvant, et al., J. Immunother. 35 (2012) 409-417. DOI:10.1097/CJI.0b013e3182594352 |
| [102] |
P. Huang, J. Zhao, C. Wei, et al., Biomater. Sci. 5 (2016) 120-127. |
 2021, Vol. 32
2021, Vol. 32