As one of twenty essential amino acids, cysteine (Cys) plays a pivotal role in numerous physiological processes involving protein synthesis and effective equilibrium of oxidation-reduction potential in vivo. The intracellular concentration and metabolism abnormity of Cys are closely related to some diseases like liver damage, breast cancer, Alzheimer's and Parkinson's diseases [1-3]. However, limited by the scarcity of efficient testing means of intracellular Cys and Cys containing proteins at present, pharmacological and pathological study is still in the fuzzy state. Besides, Cys is permitted in the production of foods, flavors, pharmaceuticals and cosmetics [4]. Therefore, many efforts have been devoted to the development of simple and reliable methods for efficient detection techniques of Cys [5]. Among various sensing strategies, fluorimetry has the unique advantages of being non-invasive and orthogonal to other process with very few side effects, low cost, simple manipulation, excellent reproducibility and superb sensitivity. Diverse design methods of fluorescent probes for detection tools of Cys have been reported recently, such as native chemical ligation reaction, cyclization reaction with aldehyde groups, aromatic nucleophilic substitution rearrangement reaction, addition-cyclization with acrylates, Michael addition, the disulfide or other covalent bonds breaking, deprotection reaction of dinitrobenzene sulfonyl and others [5-16]. Nevertheless, most of these methods are used for the preparation of small organic molecules whose quenchers make direct connections to fluorophores [6, 17-19]. They are accompanied by irreconcilable trouble of sophisticated multi-step synthesis, low yield and complicated separation process.
It is known that traditional fluorophores suffer from detrimental aggregation caused quenching (ACQ) phenomenon. Aggregation induced emission (AIE) effect was put forward by Tang's group in 2001, which offered a possibility to elegantly overcome this bothersome issue based on restriction of intramolecular motion (RIM) mechanisms [20]. The last decade has witnessed the flourishing and booming of developing a series of AIE molecules and macromolecules with facilitating practical applications ranging from chemical sensors to biological probes and optoelectronic devices [21-28]. Whereas, such molecules usually show poor solubility in physiological circumstance due to conjugate rigid structures (such as aromatic rings) besides complex preparation. In consequence, organic solvents are employed to dissolve them as well as practical applications are badly astricted [29-38]. For example, a colorimetric and turn-on AIE probe SATZ was used for detecting Cys and homocysteine (Hcy) with a large Stokes shift in phosphate-buffered saline (PBS) buffer-dimethyl sulfoxide (DMSO) [39]. Organic solvents are not appropriate for biological applications because of toxicity and biocompatibility. Thus, the facile construction of water-soluble AIE probe with excellent selectivity and sensitivity is significantly important but rarely reported [40].
To solve this problem, we reported a study on water-soluble fluorescent probe to efficiently detect the Cys in situ. The synthetic route of the fluorescent probe consisted of two distinct parts is illustrated in Scheme 1. The first is the negatively charged TPE-2SO3Na with the strong polar anionic sulfonate groups that acted as the hydrophilic sites. TPE-1, TPE-2, TPE-3 and TPE-4 were achieved according to the previous literature, confirmed by NMR and HRMS spectra [41-43]. TPE-4 was generated to TPE-2SO3Na by reacting with 1,3-propane sultone in the presence of sodium ethoxide. The other was the amphiphilic polymer poly(DNBS-MMA-co-PEGMA) containing the hydrophilic PEGMA segments, a typical nonionic water-soluble monomer. The monomer DNBS-MMA was synthesized by a one-step nucleophilic substitution reaction between 2-aminoethyl methacrylate hydrochloride and 2,4-dinitrobenzenesulfonyl chloride with high yield. The precursor polymer was prepared through simple radical polymerization and the mole percentage of DNBS-MMA as a triggered moiety was evaluated to 34.5% on the basis of 1H NMR spectrum, approximate to the proportion of monomers. Sufficient polyethylene glycol segments endowed the good water solubility to the precursor polymer. As showed in Fig. S5 (Supporting information), the number average molecular weight and molecular weight distribution of the random copolymer poly(DNBS-MMA-co-PEGMA) were measured by virtue of GPC method. In addition, quenchers and fluorophores were individuals which were synthesized alone and avoided a complex synthesis process. Detailed synthesis and characterization data were displayed in the Experimental Section and Supporting information (Figs. S1-S5 in Supporting information). Taken together, all experimental results were satisfying and coincided with the expected values.

|
Download:
|
| Scheme 1. Synthetic routes of TPE-2SO3Na and poly(DNBS-MMA-co-PEGMA). | |
When polylysine was added into TPE-2SO3Na aqueous solution, the attractive fluorescence intensity corresponding to the maximum peak at 630 nm also enhanced dramatically (Fig. S6 in Supporting information). The typical AIE behaviors were ascribed to the electrostatic interaction of between anions and cations causing the restriction of the intramolecular rotation, which had been extensively investigated [44-48]. This basic principle provided an idea to detect some species by producing one kind of compound possessing electrically charges to induce fluorescence of another molecule bearing the opposite charges, which incorporated stimuli response into the emission process.
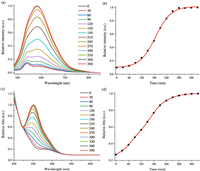
To verify this hypothesis, UV–vis absorption and fluorescence emission spectral methods were used to monitor desirable changes, since Cys could react with poly(DNBS-MMA-co-PEGMA) to generate aliphatic amine groups. Emission and absorption spectra measured every 30 min, as could be seen from Fig. 1. At the beginning, although protonated amine groups continuously formed, their contents were not enough to electrostatically interact with TPE-2SO3Na. AIE intensity was negligible excitated at 490 nm. As time increased, positively charged amine contents of the polymer increased a lot, resulting in the marked increment of red-emissive response ranging from 550 nm to 850 nm. Comparing with shorter-wavelength excitation, such as UV light, 490 nm showed much suitable for organisms and polymers as a result of improved photon penetration and minimized permanent damage to both samples to be measured as well as the assay systems [49]. At the same time, red fluorescence also avoided the biological autofluorescence and decreased interference of macromolecular clusters in the analyte, which was the key factor for lower background signal and higher signal-to-noise ratio. After 300 min, the relative absorption band centered at 352 nm had almost ground to a halt. The whole time course experiments investigated a balance of electrostatic equilibrium in good agreement with fluorescence spectral data and proved aforementioned prediction. The maximum emission intensity at 632 nm was verified to be 10 times than the original value. As a consequence, the facile approach suggested new opportunities to design, optimize, and fabricate Cys probes.
|
Download:
|
| Fig. 1. Time course of (a) emission and (c) absorption spectra of TPE-2SO3Na (0.005 mg/mL) and poly(DNBS-MMA-co-PEGMA) (0.031 mg/mL) in PBS buffer in the presence of Cys (0.0602 mg/mL) at 37 ℃. (b) The relative emission intensity (I/I0) at 632 nm and (d) the relative absorption intensity (I/I0) at 352 nm of the above system as a function of time. Excitation wavelength: 490 nm. | |
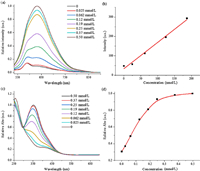
As demonstrated in Fig. 2a, fluorescence titrations were performed in PBS buffer after incubation the two-ingredient probe with various contents of Cys at 37 ℃. In order to reduce the windage of measurement, the concentrations of AIE-active fluorophore TPE-2SO3Na and poly(DNBS-MMA-co-PEGMA) were set as 0.005 and 0.031 mg/mL, respectively. Within expectation, incremental amounts of Cys also conduced to the improvement of emission. After the concentration of Cys was over 0.25 mmol/L, the increasing amplitude of intensity slowed down. It meant the saturation was achieved. A working relationship between relative emission intensity (I/ I0) at 632 nm and concentrations of Cys was established in Fig. S7 (Supporting information). Additionally, absorption titrations were also explored under the same conditions described above with similar results (Figs. 2c and d). The strong absorption band at 352 nm gradually increased along with the increasing Cys concentration up to 0.25 mmol/L. Fluorescence intensity at 632 nm showed good linear increase in a range from 0 nmol/L to 190 nmol/L, so that it was considered to high accuracy of quantification. The detection limit was estimated to 73 nmol/L (Detection Limit=3k/s, k is the standard deviation of blank sample, s is the slope utilizing straight line fitting method in Fig. 2b), indicating remarkable sensitivity. Low concentration of Cys could be effectively detected based on this probe.
|
Download:
|
| Fig. 2. (a) Emission and (c) absorption spectra of TPE-2SO3Na (0.005 mg/mL) and poly(DNBS-MMA-co-PEGMA) (0.031 mg/mL) in PBS buffer in the presence of various Cys concentrations at 37 ℃. (b) Linear relationship (R2 = 0.99) between emission intensity and Cys concentrations ranging from 0 nmol/L to 190 nmol/L. (d) The relative absorption intensity (I/I0) at 352 nm of the above system as a function of Cys concentrations. Excitation wavelength: 490 nm. | |
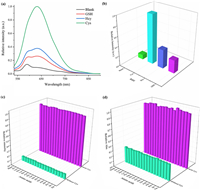
Inspired by the success in high-efficiency detection of Cys, the AIE probe was incubated in PBS buffer in the presence of various biothiols (GSH, Hcy, Cys, 0.50 mmol/L) to unveil the selectivity of the fluorogenic probe toward three main biothiols mentioned in Fig. 3a, Figs. S8 and S9 (Supporting information). On the premise of the same test conditions, intensity of the probe after incubation of Cys was four or three times than that of GSH or Hcy, as illuminated in Fig. 3b. Intensity changes provided great convenience for distinguishing these biothiols in most cases. The mechanisms of selectivity are as follows. The pKa values of Cys and Hcy thiolate anions are 8.3 and 10.0, respectively [50]. In the same weakly alkaline environment, the thiolate/thiol ratio of Hcy is lower than Cys. The reactivity of Cys is higher than Hcy, which resulted in brighter fluorescence after addition of Cys. The thiol of GSH is located in the middle of the tripeptide and the great steric hindrance reduces response to dinitrobenzenesulfonate (DNBS). As a result, its corresponding fluorescence was weakest among the three biothiols. These facts endow the red-emissive assay for Cys detection in aqueous medium with excellent selectivity.
|
Download:
|
| Fig. 3. (a) Emission spectra of TPE-2SO3Na (0.005 mg/mL), and poly(DNBS-MMA-co-PEGMA) (0.031 mg/mL) in PBS buffer in the presence of various biothiols (0.50 mmol/L) at 37 ℃ for 390 min. (b) The histogram of the relative emission intensity (I/I0) at 632 nm of the above system. The histogram of the (c) relative emission intensity (I/I0) at 632 nm and (d) relative absorption intensity (I/I0) at 352 nm of TPE-2SO3Na (0.005 mg/mL), and poly(DNBS-MMA-co-PEGMA) (0.031 mg/mL) in PBS buffer in the presence of various amino acids (0.50 mmol/L Ser, Leu, Met, Lys, Phe, Asp, Gly, Val, Gln, His, Glu, Arg, Ala, Pro, Ile, Thr, "Blank" for only Cys) with or without Cys (0.50 mmol/L) at 37 ℃. Excitation wavelength: 490 nm. | |
In addition, to further appraise the specific nature of the probe for Cys, a wide variety of natural amino acids (Ser, Leu, Met, Lys, Phe, Asp, Gly, Val, Gln, His, Glu, Arg, Ala, Pro, Ile, Thr) were tested and analyzed in the competing experiments. From Figs. 3c and d, emission or absorption maxima of each spectra seemed to only make a difference with Cys concentration. The data unambiguously denoted that the probe did not respond to other amino acids. Corresponding spectra overlapped completely, as provided in Fig. S10 (Supporting information). As a result, it has been demonstrated that these amino acids had nearly no interference on the AIE-based emission enhancement. This probe offered selective detection because of DNBS.
To clarify mechanism of turn on fluorescence of this superb probe, polylysine was used to replace poly(DNBS-MMA-co-PEGMA) and Cys. As described in Fig. S6 (Supporting information), the emission peak maximum of TPE-2SO3Na and polylysine was 630 nm, a small shift (~2 nm) compared with that of assay system. It could be deduced that generated amine groups were crucial to the detection process. Cys could cleave DNBS groups from electroneutral poly(DNBS-MMA-co-PEGMA) to afford poly(MMA-co-PEGMA). Aliphatic amine groups in PBS buffer were protonated (pKa ≈ 10.5), the formed positive centers (NH3+) could give electrostatic interactions with dissociative TPE-2SO3Na [51]. Therefore, rotations of benzene rings of TPE-2SO3Na were restricted and the emission was intensified. This inference was inspected by NMR spectra (Fig. S11 in Supporting information). The resonance at 8.67, 8.58 and 8.35 ppm corresponding to the protons at dinitrobenzene groups, nearly disappeared. As a result, bare amine groups appeared. This fact also identified the mechanism in accordance with previous literatures [44, 52-54]. In general, this process initiated with formation of protonated amine groups followed by Coulomb attraction between polycation and negatively charged TPE-2SO3Na. The maxima of the assay system differed from that of aggregates (657 nm) because of dissimilar states of aggregation (Fig. S12 in Supporting information).
Moreover, emergence of amine groups depended on the nucleophilic substitution of DNBS segments by thiolate, which closely related to thiolate/thiol ratio. However, thiolate/thiol ratios were susceptible to pH value. To examine the pH effect on the reliable detection process, fluorimetric studies involving varying pH values were conducted. The four pH, 6.6, 7.0, 7.4, 7.8, were selected, which were involved in the most physiological conditions. As presented in Figs. S13 and S14 (Supporting information), it could be postulated that the lower the pH, the less the thiolate/thiol ratio, the lower reactivity and the weaker fluorescence. The maximum emission peak at 632 nm versus reaction time in the presence of varying pH values was plotted in Fig. S14b (Supporting information). The high pH, such as 7.4, had advantages of short reaction time (less than 120 min), holding great potential for extensive real-world applications. UV spectrophotometry also uncovered the similar results in Fig. S15 (Supporting information).
In summary, an organic solvent-free fluorescent probe for cysteine was synthesized and fully characterization. The fundamental issues on emission of aggregation state and large enough Stokes shift of 142 nm were well resolved by AIE strategy. TPE with electro-withdraw substituents took the intrinsic advantages of red-emissive AIE fluorogens. The latter with a fluorescence range of 550–850 nm made it possible in vivo imaging, which was not affected by significant scattering and autofluorescence of biological tissue. The TPE-2SO3Na was synthesized by connecting TPE-4 moiety and sodium alkyl sulfonate groups, attaching competent solubility in water without the help of any organic solvents. Amphiphilic poly(DNBS-MMA-co-PEGMA) could transform to polyelectrolytes in the presence of Cys. Then the electrostatic interaction between the obtained positively charged polymer and negatively charged TPE would lead to the obviously fluorescence increase. Double responses in fluorescence and ultraviolet-visible spectra also made it convenient for practical applications. Cys could be detected from three common biothiols with mild conditions, high selectivity and sensitivity. At the same time, common amino acids did not interfere with the test, which brought great benefits. The detection limit was as low as 73 nmol/L. It could be concluded that the ingenious combination of all the desired features into the two-component probe made it ideal for sensitive detection and quantification of Cys for most purposes, especially in-situ monitoring of Cys in water.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThe authors gratefully acknowledge financial support from the National Key Research and Development Program of China (No. 2017YFA0701303) and the National Natural Science Foundation of China (Nos. 51873097 and 21674058).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.047.
| [1] |
S. Shahrokhian, Anal. Chem. 73 (2001) 5972-5978. DOI:10.1021/ac010541m |
| [2] |
M.T. Heafield, S. Fearn, G.B. Steventon, et al., Neurosci. Lett. 110 (1990) 216-220. DOI:10.1016/0304-3940(90)90814-P |
| [3] |
J. Lin, I.M. Lee, Y. Song, et al., Cancer Res. 70 (2010) 2397. DOI:10.1158/0008-5472.CAN-09-3648 |
| [4] |
J. Wang, H. Wang, Y. Hao, et al., Food Chem. 262 (2018) 67-71. DOI:10.1016/j.foodchem.2018.04.084 |
| [5] |
X. Chen, Y. Zhou, X. Peng, J. Yoon, Chem. Soc. Rev. 39 (2010) 2120-2135. DOI:10.1039/b925092a |
| [6] |
C.X. Yin, K.M. Xiong, F.J. Huo, J.C. Salamanca, R.M. Strongin, Angew. Chem. Int. Ed. 56 (2017) 13188-13198. DOI:10.1002/anie.201704084 |
| [7] |
M. Zhang, M. Yu, F. Li, et al., J. Am. Chem. Soc. 129 (2007) 10322-10323. DOI:10.1021/ja073140i |
| [8] |
X.F. Yang, Q. Huang, Y. Zhong, et al., Chem. Sci. 5 (2014) 2177-2183. DOI:10.1039/c4sc00308j |
| [9] |
D. Zhang, Z. Yang, H. Li, et al., Chem. Commun. 52 (2016) 749-752. DOI:10.1039/C5CC07298K |
| [10] |
X. Yang, Y. Guo, R.M. Strongin, Angew. Chem. Int. Ed. 50 (2011) 10690-10693. DOI:10.1002/anie.201103759 |
| [11] |
L. He, X. Yang, K. Xu, W. Lin, Anal. Chem. 89 (2017) 9567-9573. DOI:10.1021/acs.analchem.7b02649 |
| [12] |
L. Yang, H. Xiong, Y. Su, et al., Chin. Chem. Lett. 30 (2019) 563-565. DOI:10.1016/j.cclet.2018.12.017 |
| [13] |
L. Yan, Z. Kong, W. Shen, et al., RSC Adv. 6 (2016) 5636-5640. DOI:10.1039/C5RA22245A |
| [14] |
Y. Cai, J. Fang, H. Zhu, et al., Sens. Actuator. B -Chem. 303 (2020) 127214. DOI:10.1016/j.snb.2019.127214 |
| [15] |
Y.F. Kang, L.Y. Niu, Q.Z. Yang, Chin. Chem. Lett. 30 (2019) 1791-1798. DOI:10.1016/j.cclet.2019.08.013 |
| [16] |
L.Y. Niu, Y.Z. Chen, H.R. Zheng, et al., Chem. Soc. Rev. 44 (2015) 6143-6160. DOI:10.1039/C5CS00152H |
| [17] |
G. Jiang, X. Liu, Q. Chen, et al., Sens. Actuator. B -Chem. 252 (2017) 712-716. DOI:10.1016/j.snb.2017.06.066 |
| [18] |
R. Li, X. Huang, G. Lu, C. Feng, RSC Adv. 8 (2018) 24346-24354. DOI:10.1039/C8RA03756F |
| [19] |
Y. Liu, K. Xiang, B. Tian, J. Zhang, Tetrahedron Lett. 57 (2016) 2478-2483. DOI:10.1016/j.tetlet.2016.04.068 |
| [20] |
J. Luo, Z. Xie, J.W.Y. Lam, et al., Chem. Commun. (2001) 1740-1741. |
| [21] |
B. Wu, T. Xue, W. Wang, et al., J. Mater. Chem. C 6 (2018) 8538-8545. DOI:10.1039/C8TC02621A |
| [22] |
B. Wu, J. Shen, W. Wang, T. Xue, Y. He, Dyes Pigm. 170 (2019) 107569. DOI:10.1016/j.dyepig.2019.107569 |
| [23] |
B. Wu, W. Wang, J. Wang, S. Li, Y. He, Dyes Pigm. 157 (2018) 290-297. DOI:10.1016/j.dyepig.2018.04.066 |
| [24] |
Q. Luo, F. Cao, C. Xiong, Q. Dou, D.H. Qu, J. Org. Chem. 82 (2017) 10960-10967. DOI:10.1021/acs.joc.7b01877 |
| [25] |
J. Mei, Y. Huang, H. Tian, ACS Appl. Mater. Interfaces 10 (2018) 12217-12261. DOI:10.1021/acsami.7b14343 |
| [26] |
D. Ding, K. Li, B. Liu, B.Z. Tang, Acc. Chem. Res. 46 (2013) 2441-2453. DOI:10.1021/ar3003464 |
| [27] |
J. Zhao, Z. Chi, Y. Zhang, et al., J. Mater. Chem. C 6 (2018) 6327-6353. |
| [28] |
X. Zhu, J.X. Wang, L.Y. Niu, Q.Z. Yang, Chem. Mater. 31 (2019) 3573-3581. DOI:10.1021/acs.chemmater.9b01338 |
| [29] |
K. Wang, T. Leng, Y. Liu, et al., Sens. Actuator. B: Chem. 248 (2017) 338-345. DOI:10.1016/j.snb.2017.03.127 |
| [30] |
L. Peng, Z. Zhou, R. Wei, et al., Dyes Pigm. 108 (2014) 24-31. DOI:10.1016/j.dyepig.2014.04.020 |
| [31] |
J. Mei, J. Tong, J. Wang, et al., J. Mater. Chem. 22 (2012) 17063-17070. DOI:10.1039/c2jm32892e |
| [32] |
X. Tan, Y. Du, B. Yang, C. Ma, RSC Adv. 5 (2015) 55165-55169. DOI:10.1039/C5RA06244F |
| [33] |
J. Mei, Y. Wang, J. Tong, et al., Chem. Eur. J. 19 (2013) 613-620. DOI:10.1002/chem.201202969 |
| [34] |
L. Bu, J. Chen, X. Wei, et al., Dyes Pigm. 136 (2017) 724-731. DOI:10.1016/j.dyepig.2016.09.032 |
| [35] |
Y. Huang, J. Mei, X. Ma, Dyes Pigm. 165 (2019) 499-507. DOI:10.1016/j.dyepig.2019.02.053 |
| [36] |
F. Dong, H. Lai, Y. Liu, et al., Talanta 206 (2020) 120177. DOI:10.1016/j.talanta.2019.120177 |
| [37] |
Q. Chen, C. Jia, Y. Zhang, et al., J. Mater. Chem. B 5 (2017) 7736-7742. DOI:10.1039/C7TB02076G |
| [38] |
L. Yan, Y. Zhang, B. Xu, W. Tian, Nanoscale 8 (2016) 2471-2487. DOI:10.1039/C5NR05051K |
| [39] |
H. Song, Y. Zhou, H. Qu, et al., Ind. Eng. Chem. Res. 57 (2018) 15216-15223. DOI:10.1021/acs.iecr.8b04643 |
| [40] |
Z.W. Ning, S.Z. Wu, G.J. Liu, et al., Chem. Asian J. 14 (2019) 2220-2224. DOI:10.1002/asia.201900551 |
| [41] |
F. Hu, Y. Huang, G. Zhang, et al., Anal. Chem. 86 (2014) 7987-7995. DOI:10.1021/ac502103t |
| [42] |
X. He, Z. Zhao, L.H. Xiong, et al., J. Am. Chem. Soc. 140 (2018) 6904-6911. DOI:10.1021/jacs.8b02350 |
| [43] |
G. Feng, Y. Yuan, H. Fang, et al., Chem. Commun. 51 (2015) 12490-12493. DOI:10.1039/C5CC03807C |
| [44] |
C. Yu, Y. Wu, F. Zeng, et al., Biomacromolecules 14 (2013) 4507-4514. DOI:10.1021/bm401548u |
| [45] |
Y. Li, X. Hu, S. Tian, et al., Biomaterials 35 (2014) 1618-1626. DOI:10.1016/j.biomaterials.2013.10.077 |
| [46] |
X. Liu, Y. Zeng, J. Liu, et al., Langmuir 31 (2015) 4386-4393. DOI:10.1021/acs.langmuir.5b00155 |
| [47] |
W. Chen, Q. Li, W. Zheng, et al., Angew. Chem. Int. Ed. 53 (2014) 13734-13739. DOI:10.1002/anie.201407606 |
| [48] |
J. Chen, Y. Wang, W. Li, et al., Anal. Chem. 86 (2014) 9866-9872. DOI:10.1021/ac502496h |
| [49] |
Y. He, Y. Zhong, Y. Su, et al., Angew. Chem. Int. Ed. 50 (2011) 5695-5698. DOI:10.1002/anie.201004398 |
| [50] |
A.V. Glushchenko, D.W.J.A. Jacobsen, Antioxid. Redox Signaling 9 (2007) 1883-1898. DOI:10.1089/ars.2007.1809 |
| [51] |
K. Dhara, Y. Hori, R. Baba, K. Kikuchi, Chem. Commun. 48 (2012) 11534-11536. DOI:10.1039/c2cc36591j |
| [52] |
V.G. Naik, S.D. Hiremath, A. Das, et al., Mater. Chem. Front. 2 (2018) 2091-2097. DOI:10.1039/C8QM00417J |
| [53] |
S. Long, Q. Qiao, L. Miao, Z. Xu, Chin. Chem. Lett. 30 (2019) 573-576. DOI:10.1016/j.cclet.2018.11.031 |
| [54] |
Y. Wu, J. Wen, H. Li, S. Sun, Y. Xu, Chin. Chem. Lett. 28 (2017) 1916-1924. DOI:10.1016/j.cclet.2017.09.032 |
 2021, Vol. 32
2021, Vol. 32 

