b School of Physical Science and Technology, Shanghai Tech University, Shanghai 201210, China;
c University of Chinese Academy of Sciences, Beijing 100049, China
Wheel-like supramolecular rings are one of the most striking molecules, because of their aesthetically pleasing structure, unique physicochemical properties, such as magnetism, catalysis and gas adsorption ability [1-8]. Such molecules generally show much structural diversity and tunability, owing to their formation processes which are self-assembly of diverse secondary building units (SBUs). The famous wheel-shaped molecules of {Mo154}, {Mo176} and gigantic chiral molybdenum blue wheel {Mo124Ce4} are all constructed from SBUs of {Mo8} and {Mo2} [1, 2, 9-11]. The series of "Ferric Wheels" compounds are mostly consisting of several repeating units of dinucleariron [12-14]. The crucial point of designing and synthesizing of supramolecular wheels is the selection of suitable SBUs.
Complexes of bis-dimethylglyoximate of transition metals as efficient catalysts, have been widely investigated in recent years [15, 16] While the metal-including compounds of tris-dimethylglyoximate or tris-dioximates are in deficiency (Fig. 1a), on account of their ligands fully wrapping metal center. However, these complexes can form a clathrochelate through self-assembly of capping on both ends of the molecule [17-20]. Recently, many functionalized boron-capped clathrochelate complexes have been used as metalloligands for the assembly of cage-like molecules [21, 22]. It is possible that clathrochelate of tris-dimethylglyoximate could also be used as SBU for the construction of supramolecular structures.

|
Download:
|
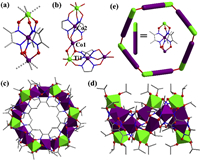
| Fig. 1. The structure of (a) Co(Dmg)3 and (b) Ti-capped cobalt clathrochelate compound 1 (Ti: bright green, Co: purple, Cl: green, N: blue, O: red, C: gray). | |
Here, we successfully synthesize a wheel-like heterometallic structure comprised of six repetitive units of titanium-capped cobalt clathrochelates. In addition, the packing modes of these wheel molecules are largely dependent on the guest molecules, which further impact their gas adsorption properties.
At the beginning of our study, compound [CoTi2Cl6(Dmg)3] (1, Dmg=dimethylglyoxime) was obtained by the reaction of TiCl4, cobalt(II) acetate and dimethylglyoxime in the presence of 4-dimethylaminopyridine (DMAP) or melamine in acetonitrile solvent. The Ti4+ ions in the structure of 1 was coordinated with three chloride ions and capped by a {Co(Dmg)3} component through oxo-bridged (Fig. 1b). The successful synthesis of this molecule proves that structural units of [CoM2(Dmg)3] (M=transition metal ions) could be stably presented and may be further used as a subunit for the construction of supramolecular wheel structures. Unfortunately, the structure of compound 1 is very unstable. The hydrolysis of compound 1 begins when it is removed from the mother liquor. Thus it could not be used as precursor for a stepwise assembly.
However, we successfully obtained a ring-molecule [C6H15N4]2[TiCo2(μ2-Oipr)(Oipr)2(Dmg)3]6 (2, Oipr=isopropyl) by changing the solvent and titanium source of the reaction system. Single-crystal X-ray diffraction analysis revealed that compound 2 contained a wheel unit [Co2Ti(μ2-Oipr)(Oipr)2 (Dmg)3]6 which was constructed from six SBUs of [TiCo2(Dmg)3]. The molar ratio of Ti and Co in 2 was confirmed to be about 1:2 by ICP-AES analysis (Ti: 6.60%, Co: 15.98%). In the structure of 2, the protonated hexamethylenetetramine cations surround the anionic wheel. The capping atoms of the clathrochelate of tris-dimethylglyoximate in 1 were all titanium atoms, while the capping atoms in each SBU of the wheel 2 were divided into two kinds: six-coordinated Ti (named Ti1) and five-coordinated Co (named Co1). Each {TiCo2(Dmg)3} unit is further linked to two adjacent ones by sharing {TiCoO2} units (Figs. 2a and b). The Co-O bond lengths of Co1 are 1.992 Å, 2.048 Å, 2.070 Å, 2.004 Å and 2.090 Å, and the Ti-O bond lengths of Ti1 are 2.065 Å, 2.018 Å, 2.147 Å, 1.986 Å, 1.816 Å and 1.795 Å, respectively. Bond valence sum (BVS) calculations of Ti1 and Co1 show that they are Ti(IV) and Co(II) (Table S2 in Supporting information) [23]. The molecular shape of 2 is the classical 6-membered ring with chair-like configuration (Figs. 2c–e). The constructed [Ti6Co12] rings in 2 are further packed into beautiful supramolecular framework with 1-dimensional channels along c direction (Figs. 3a and c). In the supramolecular framework of 2, the hexamine guest exists in the gap of three rings (Figs. 3a and c). The diameter of the wheel is about 0.6 nm. As hexamine occupies a part of the voids, the pore volume ratio of this framework is 9.9% calculated by PLATON [24].
|
Download:
|
| Fig. 2. (a) The SBU of the wheel cluster 2. (b) The link between two {TiCo2(Dmg)3} units, with the methyl groups of the dimethy-lglyoxime ligands omitted for clarity. (c, d) The ring structure of 2 and (e) simplified molecular model of 2. Coordination spheres of Ti and Co atoms are shown as polyhedra for clarity (Ti: bright green, Co: purple, Cl: green, N: blue, O: red, C: gray). | |

|
Download:
|
| Fig. 3. The packing of 2 viewed along the (a) b direction and (c) c direction. The packing of 3 viewed along the (b) b direction and (d) c direction. Coordination spheres of Ti and Co atoms are shown as polyhedra for clarity (Ti: bright green, Co: purple, Cl: green, N: blue, O: red, C: gray). | |
The structure and solvent accessible volume ratio of frameworks are often influenced by guest molecules in their pores. When we chose another organic amine of DMAP as the structure-directing agent in the synthesis of 2, compound 3H6[TiCo2(μ2-Oipr)(Oipr)2(Dmg)3]6 with new packing mode of these [Ti6Co12] rings was prepared. The Ti: Co ration in 3 was also confirmed to be about 1:2 by ICP-AES analysis (Ti: 6.98%, Co: 17.20%). The X-ray diffraction analysis shows that compound 3 crystallizes in a monoclinic space group, P21/n, which has lower symmetry than compound 2. The packing structure of 3 becomes loser and emerges more intermolecular spaces (Figs. 3b and d). And, there are not organic ammoniums found around [Ti6Co12] rings. Therefore, the cations of compound 3 that surround the [Ti6Co12] anions for charge balance would be H+. Furthermore, the framework of 3 exposes more voids with the pore volume ratio of 25.9%, excluding guest molecules, as calculated by PLATON [24]. Possibly, the guests in voids of 3 might be some disordered isopropanol molecules.
Solid-state UV–vis light absorption analysis and infrared spectrum analysis were carried out on the samples of 2 and 3. The UV–vis spectra of 2 and 3 were very similar. Both of them have significant absorption in the visible range which was decreased with wavelength increasing. An absorption peak was also presented in IR range, which should be contributed to the d-d transition of cobalt ions (Figs. S7 and S8 in Supporting information). The FT-IR spectra of 2 and 3 are almost the same, which further prove that they are composed of the same [Ti6Co12] building units. The peaks at 2964 and 1578 cm−1 of 2 maybe belong to the vibrations of the -CH3 and -CN- groups, respectively. The peaks around 1260−1050 cm−1 of 2 should be inherent stretching vibrations of the C-O groups. Similar peak assignment could also be made for 3. The results of thermogravimetric analyses (TGA) of 2 and 3 confirm that 3 has more weight loss of 16.3% than 5.4% of 2 from 20 ℃ to 150 ℃ (Figs. S3 and S4 in Supporting information), corresponding to the release of small guest molecules that could not be crystallographically located.
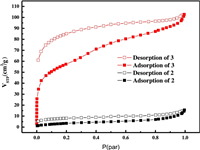
As demonstrated above, different organic amine agents lead to the difference in the packing structures of [Ti6Co12] rings. Usually, the compound with higher ratio of pore volume will possess better capability of gas adsorption. The N2 adsorption isotherms for activated samples of 2 and 3 were collected. It can be seen from Fig. 4 that the N2 adsorption of 2 is very limited, and the maximum N2 uptake at 1bar for 2 is only 12.3 cm3/g. However, the maximum N2 uptake at 1 bar for 3 is increased to 102.8 cm3/g, which has a BET surface area of 199.4 m2/g and Langmuir surface area of 292.0 m2/g. The N2 Isotherms of compound 3 exhibited type-I adsorption/desorption behaviors with significant sorption hysteresis, suggesting the permanent microporosity. The results are in good agreement with the looser packing structure of 3.
|
Download:
|
| Fig. 4. N2 adsorption isotherms for 2 and 3 recorded at 77 K, respectively. | |
In summary, an unusual [Ti6Co12] ring with titanium-linked cobalt clathrochelates has been synthesized and characterized. We also realized different packing structures of these rings by changing the applied synthetic conditions, and finally porous frameworks based on such [Ti6Co12] rings were formed. This work indicates that the titanium-capped cobalt clathrochelates, [CoM2(Dmg)3], could be used as a versatile building unit for the assembly of heterometallic Ti-Co complexes.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work is supported by the National Natural Science Foundation of China (NSFC, Nos. 21673238 and 21922111), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20000000).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.04.004.
| [1] |
A. Müller, E. Krickemeyer, J. Meyer, et al., Angew. Chem. Int. Ed. 34 (1995) 2122-2123. DOI:10.1002/anie.199521221 |
| [2] |
H. Imai, T. Akutagawa, F. Kudo, et al., J. Am. Chem. Soc. 131 (2009) 13578-13579. DOI:10.1021/ja9048042 |
| [3] |
H.N. Miras, G.J. Cooper, D.L. Long, et al., Science 327 (2010) 72-74. DOI:10.1126/science.1181735 |
| [4] |
H. Zheng, M.H. Du, S.C. Lin, et al., Angew. Chem. Int. Ed. 130 (2018) 11142-11145. DOI:10.1002/ange.201806757 |
| [5] |
X.Y. Zheng, Y.H. Jiang, G.L. Zhuang, et al., J. Am. Chem. Soc. 139 (2017) 18178-18181. DOI:10.1021/jacs.7b11112 |
| [6] |
S. Noro, R. Tsunashima, Y. Kamiya, et al., Angew. Chem. Int. Ed. 48 (2009) 8703-8706. DOI:10.1002/anie.200903142 |
| [7] |
L.Q. Jared, S.W.P. Chen, N. Hiroyuki, et al., Angew. Chem. Int. Ed. 56 (2017) 1-5. DOI:10.1002/anie.201610955 |
| [8] |
C.W. Zhao, Y.Z. Han, S. Dai, et al., Angew. Chem. Int. Ed. 56 (2017) 16252-16256. DOI:10.1002/anie.201709096 |
| [9] |
A. Müller, P. Gouzerh, Chem. Soc. Rev. 41 (2012) 7431-7463. DOI:10.1039/c2cs35169b |
| [10] |
W.M. Xuan, R. Pow, N. Watfa, et al., J. Am. Chem. Soc. 141 (2019) 1242-1250. DOI:10.1021/jacs.8b09750 |
| [11] |
W.M. Xuan, R. Pow, D.L. Long, L. Cronin, Angew. Chem. Int. Ed. 56 (2017) 9727-9973. DOI:10.1002/anie.201702957 |
| [12] |
K.L. Taft, S.J. Lippard, J. Am. Chem. Soc. 112 (1990) 9629-9630. DOI:10.1021/ja00182a027 |
| [13] |
O.L. Sydora, P.T. Wolczanski, E.B. Lobkovsky, Angew. Chem. Int. Ed. 42 (2003) 2685-2687. DOI:10.1002/anie.200351143 |
| [14] |
A.H. Abu-Nawwas, J. Cano, P. Christian, et al., Chem. Commun. 3 (2004) 314-315. |
| [15] |
P.W. Du, J. Schneider, G.G. Luo, W.W. Brennessel, R. Eisenberg, Inorg. Chem. 48 (2009) 4952-4962. DOI:10.1021/ic900389z |
| [16] |
J.J. Zhong, C.J. Wu, Q.Y. Meng, et al., Adv. Synth. Catal. 356 (2014) 2846-2852. DOI:10.1002/adsc.201400588 |
| [17] |
Y.Z. Voloshin, O.A. Varzatskii, A.S. Belov, et al., Polyhedron 27 (2008) 325-334. DOI:10.1016/j.poly.2007.09.008 |
| [18] |
W.J. Liu, W.J. Huang, M. Pink, D. Lee, J. Am. Chem. Soc. 132 (2010) 11844-11846. DOI:10.1021/ja104038s |
| [19] |
A.S. Belov, A.V. Vologzhanina, Y.V. Fedorov, et al., Inorg. Chem. Commun. 35 (2013) 242-246. DOI:10.1016/j.inoche.2013.06.036 |
| [20] |
A.V. Dolganov, A.S. Belov, V.V. Novikov, et al., Dalton Trans. 42 (2013) 4373. DOI:10.1039/c3dt33073g |
| [21] |
G. Cecot, B. Alameddine, S. Prior, et al., Chem. Commun. 52 (2016) 11243-11246. DOI:10.1039/C6CC06066H |
| [22] |
S. Jansze, G. Cecot, M.D. Wise, et al., J. Am. Chem. Soc. 138 (2016) 2046-2054. DOI:10.1021/jacs.5b13190 |
| [23] |
N.E. Brese, M. Okeeffe, Acta Crystallogr. B 47 (1991) 192-197. DOI:10.1107/S0108768190011041 |
| [24] |
P. Vandersluis, A.L. Spek, Acta Crystallogr. A 46 (1990) 194-201. DOI:10.1107/S0108767389011189 |
 2021, Vol. 32
2021, Vol. 32 

