b Jiangsu Provincial Key Laboratory for Advanced Carbon Materials and Wearable Energy Technologies, Soochow University, Suzhou 215006, China
As the most popular energy storage device, lithium polymer batteries (LPBs) have earned wide research interests in applications such as electronic devices and electric vehicles in pursuit of thinner, lighter and safer energy storage [1, 2]. Tremendous researches have been carried out to improve the specific capacity, cycle life, cell safety and energy density of LPBs [3]. Despite numerous studies on continuously improving the capacity of the electrode and energy density of the batteries, the choices of separators that prevent the cell from short-circuit while allow ion migration are quite limited. The separator plays an important role in underpinning the electrochemical reaction kinetics for improved cell performance including cycle stability, energy density and power density [4, 5]. High quality separators should have high thermo-stability to resist the heat generated during the battery operation and good electrolyte adsorption to accommodate sufficient electrolyte. The physicochemical properties and stability of the separator is thus critical to the safety and life of batteries.
Current commercialized separators typically are polyolefin-based separators made from polyethylene (PE), polypropylene (PP), or blends thereof. However, a compromise must be made between the safety and the electrochemical performance of LPBs owing to their low melting point and in sufficient electrolyte uptake. Gel polymer electrolytes (GPEs) formed by the gelation of polymeric electrospun films as the replacement of conventional separators have emerged to improve the ionic conductivity, electrolyte uptake and thermal stability [6-10]. So far, polymers used for GPEs are mainly limited to polyvinylidene fluoride (PVDF) [11, 12], polyacrylonitrile (PAN) [13], polymethyl methacrylate (PMMA) [14] and polyvinylidene fluoride-co-hexafluoro-propylene (PVDF-co-HFP) [15-18], etc. PAN-based GPEs with good flame retardancy and chemical stability have been reported to inhibit lithium dendrites upon repeated lithiation/delithiation processes [19]. Nevertheless, these polymers often present high crystallinity and poor mechanical strength in a gel form. Inorganic additives, including Al2O3, TiO2 and SiO2, were thus adopted to improve the chemical and mechanical properties of GPEs [20-22]. However, these non-porous oxides embedded in the polymer matrix might jeopardize the electrolyte adsorption. Alternately, the use of metal organic framework (MOF) materials with unique interconnected pore structure and superb absorptivity as additive in GPEs would significantly promote the electrolyte uptake and ionic conductivity [23-26]. For instance, Bai et al. used a MOF-based separator to selectively sieve Li+ for promoting the ion migration in lithium sulfur batteries [27]. Zhong et al. coated MIL-125-Ti on the Celgard separator to suppress lithium dendrite growth and extend the cycle life of lithium metal batteries [23].
Herein, we report the preparation of a composite GPE based on nanofibrous electrospun-polyacrylonitrile (PAN) membrane filled with nanocubes of iron-nickel-cobalt trimetal Prussian blue analogues (PBA) of high chemical stability. The secondary building units of transition metals in PBA located at the cubic nodes are capable of profiting both ion infiltration and electron transportation [28-31]. The trimetal PBA can be easily synthesized in fine particles with appropriate size serving as a structural filler. Thereby, the as-obtained electrospun PBA@PAN films showed greatly improved porosity, electrolyte uptake and ionic conductivity. By adjusting the content of PBA, the best physical and chemical properties of GPEs with 10% PBA addition were demonstrated. Compared with the commercial Celgard separators, the PBA@PAN-10 GPE showed better performance in lithium iron phosphate (LiFePO4) batteries in terms of specific capacity and cycle stability.
Trimetal PBA nanocubes were synthesized by the following procedure: 3 mmol of nickel(II) nitrate hexahydrate (Ni2(NO3)2·6H2O), 3 mmol of cobaltous(II) nitrate hexahydrate (Co2(NO3)2·6H2O), and 9 mmol of trisodium citrate were successively dissolved in 200 mL of distilled water to prepare the salt solution, which was then mixed with equal volume of a ligand solution containing 4 mmol dihydrate potassium ferricyanide (K3Fe(CN)6) and stirred for about 10min. After standing for 24 h, the dark gray solid product was collected by centrifugation, and washed with distilled water and ethanol for three times and dried overnight at 60 ℃ under vacuum for further use. To fabricatethe PBA@PAN films, 1 g of PAN was first dissolved in 13 mL of DMF by magnetically stirring, and then PBA obtained from above was added in different ratio (10% and 20%) to obtain a uniform precursor solution for electrospinning. The prepared solution was subjected to electrospinning, in which the parameters were set to a flow rate of 0.03 mL/min, a voltage of 17 kV, and a distance of 15 cm from the tip to the collector. Subsequently, the collected electrospun membrane was dried at 60 ℃ overnight. Thus obtained fibrous membranes were designated as PBA@PAN-x, where x is the weight ratio of PBA to PAN. Electrospun PAN films without the addition of PBA were also prepared under the same condition for control studies. The membranes were cut into discs of 19 mm in diameter, with the commercial Celgard® 2500 (Celgard) separator serving as benchmark control. All membranes were fully dried under vacuum at 80 ℃ for 24 h in order to remove all water traces (Fig. S1 in Supporting information).
The LiFePO4 electrodes were prepared from a slurry containing 80 wt% of LiFePO4, 10 wt% of Super P (SP) and 10 wt% of polyvinylidene fluoride (PVDF) in 1-methyl-2-pyrrolidone (NMP) casted on the Al foil. The coated electrodes were dried at 60 ℃ for 12 h, and then punched into discs with a diameter of 10 mm for further use. More detailed experimental procedures are supplemented in the Supporting information.
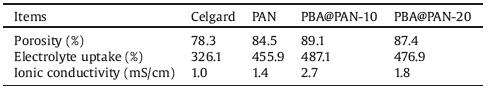
The synthesis process of PBA@PAN-x via electrospinning is schemed in Fig. 1a. The C≡N group within the molecular chains of PAN would contribute to the high ionic conductivity and mechanical strength of GPE, while the PBA nanocubes homogeneously dispersed in the nanofibers help modulate both the morphology and electrolyte wettability of the film. Fig. 1b shows the XRD patterns of PAN, the as-synthesized ternary iron-nickel-cobalt PBA, PBA@PAN-10 and PBA@PAN-20. The pattern for PBA was in good agreement with the standard profile (JCPDS No. 01-0239), indicating the successful synthesis of PBA. Moreover, it was found that the intensity of PBA diffraction peaks increases with their mass ratio in the fibers. The morphology of PBA and electrospun PBA@PAN membranes were characterized by FE-SEM and TEM. The ternary PBA nanocubes showed a uniform particle size of around 130±6 nm (Fig. 1c). Fine fibers with an average fiber diameter (AFD) of 1±0.2 μm were visualized by FE-SEM for PBA@PAN-10 (Fig. 1d), which is much larger than that of the pristine PAN fibers (0.6±0.03 μm) (Fig. S2a in Supporting information). A generally smooth surface was observed for PBA@PAN-10, in which most PBA nanocubes were probably confined inside of the PAN matrix (Fig. 1d inset). As further illustrated by TEM in Fig. 1e, PBA nanocubes were seen homogeneously dispersed in the fibers of PBA@PAN-10, which would help modulate both the morphology and electrolyte wettability of the film. By contrast, PBA@PAN-20 showed a larger AFD (> 2 μm) with more PBA nanocubes emerging on the surface, which turned the film into dark gray (Fig. S2b in Supporting information). We note the excess addition of PBA nanocubes would increase the viscosity of the electrospinning solution and further change the fiber morphology and visual appearance of PBA@PAN-20.
|
Download:
|
| Fig. 1. (a) Schematic illustration of the preparation of PBA@PAN-x. (b) XRD patterns of PAN, PBA, PBA@PAN-10 and PBA@PAN-20, respectively. (c) FE-SEM of PBA nanocubes. (d) FE-SEM and (e) TEM images of PBA@PAN-10. | |
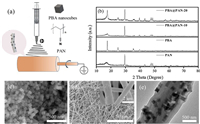
The electrolyte wettability of Celgard and composite fibers was verified by contact angle tests. Celgard showed a contact angle of ~45.4° in Fig. 2a, while PAN fibers gave greatly lowered value of ~6.5°. Notably, the addition of PBA further decreased the contract angles of PBA@PAN-x to 0°, demonstrating good electrolyte wettability of the PBA@PAN composites owing to the excellent affinity of both PAN matrix and PBA nanocubes to the electrolyte. The thermal stability of the separators is of great importance to the safety of LPBs. Celgard, PAN and PBA@PAN-x were placed in the oven at 120 ℃ overnight to evaluate their thermal stability. Compared to the fresh films before heating (Fig. 2b), the Celgard after heat treatment was curved up and became plastically deformed, while the pure PAN films lightly shrank. It is seen that PBA@PAN-10 films could tolerate the high temperature treatment without obvious deformation. Oppositely, PBA@PAN-20 became slightly more rigid with worse flexibility as a result of the excess PBA addition. It is surmised that the thinner and crumby fibers of pure PAN have lower thermal stability and mechanical strength that can easily induce deformation at high temperature. On the other hand, the addition of proper amount of PBA (10%) not only strengthen the fibers with retained flexibility, but also promotes their thermal resistance. The improved thermal stability of PBA@PAN-x could be dedicated to the safety of LPBs [27].

|
Download:
|
| Fig. 2. (a) Contact angle tests, (b) thermal stability, (c) porosity, (d) electrolyte uptake, (e) AC impedance plot and (f) ionic conductivity of Celgard, PAN and PBA@PAN-x, respectively. | |
The feasibility of the prepared films towards the electrolyte were comprehensively investigated as shown in Fig. 2 and Table 1. The porosity of the electrospun PAN and PBA@PAN-xfibers significantly exceeds that of the commercial Celgard (Fig. 2c). PBA@PAN-10 obtained the highest porosity of 89.1% owing to the synergistic contribution from the highly interconnected porous structure of electrospun fibers and appropriate amount of porous PBA. As such, greater electrolyte retention and ionic conductivity can be achieved. PBA@PAN-10 was quickly saturated with electrolyte in 15s, demonstrating a good electrolyte wettability (Fig. 2d) [32]. Remarkably, PBA@PAN-10 was able to absorb the most amount of electrolyte up to a maximum of 487.1% in contrast to those of Celgard (326.1%), PAN (455.9%) and PBA@PAN-20 (476.9%) (Table 1). It is obvious that the porous PBA helps contribute to the absorption of electrolyte and in turn gives rise to a high liquid retention rate. The more electrolyte is housed in the GPE, the less resistance there is for Li+ migration. Such a high electrolyte uptake in PBA@PAN should therefore greatly boost the ionic conductivity and lower the internal resistance of the battery. As shown in Fig. 2e, the bulk resistances (Rb) for Celgard, PAN, PBA@PAN-10 and PBA@PAN-20 fibers were measured as 6.0, 4.9, 2.7 and 3.5 Ω, corresponding to the ionic conductivity of 1.0, 1.4, 2.7 and 1.8 mS/cm, respectively (Fig. 2f). The highly porous structure of PAN-based fibers as observed from the cross-sectional FE-SEM images (Fig. S2c in Supporting information) would greatly improve the electrolyte adsorption and ionic conductivity compared to the Celgard in bulk matrix (Fig. S2d in Supporting information). Additionally, the abundant polar functional groups in the polymer chain endows the electrospun membrane with strong affinity to the electrolyte [33], which is further reinforced by the addition of appropriate amount of PBA (10%). Thus, the greatly improved ionic conductivity over Celgard would lead to fast Li+ diffusion within the PBA@PAN films and further enhanced reaction kinetics of LPBs.
|
|
Table 1 The porosity, electrolyte uptake and ionic conductivity of Celgard, PAN and PBA@PAN-x, respectively. |
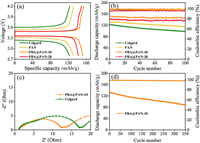
In order to examine the electrochemical performance of the fabricated PBA@PAN-x films when used for GPEs, LiFePO4 cells were assembled using lithium foil as anodes. The charge/discharge curves for Celgard and PBA@PAN-x at 0.2 C showed typical plateau of LiFePO4 cells (Fig. 3a). The initial discharge capacities of the cells with Celgard, PAN, PBA@PAN-10 and PBA@PAN-20 were 122.4, 131.4, 152.2 and 142.1 mAh/g, respectively. The highest capacity was observed from the cell with PBA@PAN-10, with a highly retained capacity of 144.9 mAh/g after 100 cycles and a highly stable Coulombic efficiency close to 100% (Fig. 3b). By contrast, the cells with Celgard and PBA@PAN-20 presented a faster capacity fading in 100 cycles. Notably, both of the PBA@PAN-x GPEs outperformed the Celgard in capacity and cycle stability. We noticed that further excessive filling of PBA into the fibers would have an opposite effect on the cell performance, showing much reduced capacity, which is probably ascribed to the non-coherent fiber morphology and in homogeneous particle distribution that impedes the movement of Li+. EIS tests on the PBA@PAN-10 cell after 100 cycles are given in Fig. 3c in comparison to Celgard. The diameter of the semicircle in the Nyquist plot reflects the interfacial resistance (Rf) of the cells [34]. It was observed that the cell with PBA@PAN-10 had a lower value of 13 Ω than the cell with Celgard, indicating a fast electrochemical kinetics in the cell with PBA@PAN-10.Fig. 3d shows the cycle stability of the PBA@PAN-10 cell at 1 C, exhibiting a low capacity decay of 0.09% per cycle in a total of 350 cycles. Overall, the electrospun PBA@PAN membranes featuring high porosity, good electrolyte wettability and improved ionic conductivity give rise to the excellent LPB performance.
|
Download:
|
| Fig. 3. Electrochemical performances of LiFePO4 cells with Celgard, PAN and PBA@PAN-x. (a) Charge/discharge vs. voltage profiles, (b) cycle stability at 0.2 C, (c) EIS spectra and (d) long life-span cycles at 1 C. | |
In summary, PBA-filled fibrous PAN membranes were fabricated by electrospinning and used as GPEs for LPBs. Compared with the commercial Celgard separators, PBA@PAN-10 possessed higher porosity due to the rich interconnected pores in the electrospun films embedding porous PBA nanocubes. The featured fibrous structure and the high absorptivity of PBA to electrolyte endow the GPE with high electrolyte uptake and ionic conductivity. Besides, the PBA@PAN composites manifest better mechanical strength and heat resistance, which is an added benefit contributing to the battery safety. LPB cells with PBA@PAN-10 as the GPEs were able to deliver a high specific capacity of 131.4 mAh/g and an ultralow capacity decay of 0.09% in a long life-span of 350 cycles at 1 C. By incorporating MOFs into the fibrous PAN network, this study paves the way for the development of high performance LPBs based on gel polymer electrolytes.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work is supported by Natural Science Foundation of China (Nos. 21805201 and 21701118), Postdoctoral Science Foundation of China (Nos. 2018T110544 and 2017M611899), Natural Science Foundation of Jiangsu Province (No. BK20170341) and the Key Technology Initiative of Suzhou Municipal Science and Technology Bureau (No. SYG201748). We also extend our sincere appreciation to the support by Suzhou Key Laboratory for Advanced Carbon Materials and Wearable Energy Technologies.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.07.008.
| [1] |
V. Etacheri, R. Marom, R. Elazari, et al., Energy Environ. Sci. 4 (2011) 3243-3262. DOI:10.1039/c1ee01598b |
| [2] |
J.B. Goodenough, K.S. Park, J. Am. Chem. Soc. 135 (2013) 1167-1176. DOI:10.1021/ja3091438 |
| [3] |
E.M. Erickson, C. Ghanty, D. Aurbach, J. Phys. Chem. Lett. 5 (2014) 3313-3324. DOI:10.1021/jz501387m |
| [4] |
S.S. Zhang, J. Power Sources 164 (2007) 351-364. DOI:10.1016/j.jpowsour.2006.10.065 |
| [5] |
M. Raja, G. Sanjeev, T.P. Kumar, A.M. Stephan, Ceramics Inter. 41 (2015) 3045-3050. DOI:10.1016/j.ceramint.2014.10.142 |
| [6] |
T.S. Yang, C.Z. Shu, Z.Q. Hou, et al., Solid State Ion. 343 (2019) 11508. |
| [7] |
T. Wu, M. Ding, C. Shi, et al., Chin. Chem. Lett. 31 (2020) 617-625. DOI:10.1016/j.cclet.2019.07.033 |
| [8] |
C. Jiang, H. Li, C. Wang, Sci. Bull. 62 (2017) 1473-1490. DOI:10.1016/j.scib.2017.10.011 |
| [9] |
B. Wang, M. Tang, Y. Wu, et al., ACS Appl. Energy Mater. 2 (2019) 5909-5916. DOI:10.1021/acsaem.9b01046 |
| [10] |
B. Wang, Y. Wu, S. Zhuo, et al., J. Mater. Chem. A 8 (2020) 5968-5974. DOI:10.1039/C9TA14239H |
| [11] |
F. Boudin, X. Andrieu, C. Jehoulet, I.I. Olsen, J. Power Sources 81 (1999) 804-807. |
| [12] |
C.Y. Chiang, M.J. Reddy, P.P. Chu, Solid State Ion. 175 (2004) 631-635. DOI:10.1016/j.ssi.2003.12.039 |
| [13] |
P. Raghavan, J. Manuel, X. Zhao, et al., J. Power Sources 196 (2011) 6742-6749. DOI:10.1016/j.jpowsour.2010.10.089 |
| [14] |
B.Y. Huang, Z.X. Wang, L.Q. Chen, et al., Solid State Ion. 91 (1996) 279-284. DOI:10.1016/S0167-2738(96)00445-6 |
| [15] |
S. Abbrent, J. Plestil, D. Hlavata, et al., Polymer 42 (2001) 1407-1416. DOI:10.1016/S0032-3861(00)00517-6 |
| [16] |
P. Raghavan, X.H. Zhao, J. Manuel, et al., Electrochim. Acta 55 (2010) 1347-1354. DOI:10.1016/j.electacta.2009.05.025 |
| [17] |
X. Li, G. Cheruvally, J.K. Kim, et al., J. Power Sources 167 (2007) 491-498. DOI:10.1016/j.jpowsour.2007.02.032 |
| [18] |
G. Cheruvally, J.K. Kim, J.W. Choi, et al., J. Power Sources 172 (2007) 863-869. DOI:10.1016/j.jpowsour.2007.07.057 |
| [19] |
M. Liu, N.P. Deng, J.G. Ju, et al., Adv. Funct. Mater. 29 (2019) 1905467. DOI:10.1002/adfm.201905467 |
| [20] |
Q.F. Peng, F. Yu, W.K. Wang, et al., Electrochim. Acta 299 (2019) 749-755. DOI:10.1016/j.electacta.2019.01.061 |
| [21] |
Y.C. Zhang, Z.H. Wang, H.F. Xiang, et al., J. Membr. Sci. 509 (2016) 19-26. DOI:10.1016/j.memsci.2016.02.047 |
| [22] |
M. Yanilmaz, Y. Lu, J.D. Zhu, X.W. Zhang, J. Power Sources 313 (2016) 205-212. DOI:10.1016/j.jpowsour.2016.02.089 |
| [23] |
Y.R. Zhong, F. Lin, M.Y. Wang, et al., Adv. Funct. Mater. (2020) 1907579. |
| [24] |
B. Kong, C. Selomulya, G. Zheng, D. Zhao, Chem. Soc. Rev. 44 (2015) 7997-8018. DOI:10.1039/C5CS00397K |
| [25] |
L.S. Fan, Z.K. Guo, Y. Zhang, et al., J. Mater. Chem. A 8 (2020) 251-258. DOI:10.1039/C9TA10405D |
| [26] |
A. Singh, R. Vedarajan, N. Matsumi, J. Electrochem. Soc. 164 (2017) H5169-H5174. DOI:10.1149/2.0191708jes |
| [27] |
S. Bai, X. Liu, Z. Kai, et al., Nat. Energy 1 (2016) 16094. DOI:10.1038/nenergy.2016.94 |
| [28] |
L. Han, X.Y. Yu, X.W. Lou, Adv. Mater. 28 (2016) 4601-4605. DOI:10.1002/adma.201506315 |
| [29] |
X. Wu, C. Wu, C. Wei, et al., ACS Appl. Mater. Interfaces 8 (2016) 5393-5399. DOI:10.1021/acsami.5b12620 |
| [30] |
Y. You, X.L. Wu, Y.X. Yin, Y.G. Guo, Energy Environ. Sci. 7 (2014) 1643-1647. DOI:10.1039/C3EE44004D |
| [31] |
Y. You, X. Yu, Y. Yin, et al., Nano Res. 8 (2014) 117-128. |
| [32] |
X. Zhao, D.S. Kim, P. Raghavan, et al., Phys. Scr. T 139 (2010) 014036. |
| [33] |
H.S. Min, J.M. Ko, D.W. Kim, J. Power Sources 119 (2003) 469-472. |
| [34] |
B. Zhao, X. Lu, Q. Wang, et al., Chin. Chem. Lett. 31 (2020) 831-835. DOI:10.1016/j.cclet.2019.06.009 |
 2021, Vol. 32
2021, Vol. 32