Pressure sensitive adhesives (PSAs) are a type of soft polymeric materials that can be reversibly sticked on different surfaces (adherends) under a slight external pressure, and have found wide applications in our daily life, such as self-adhesive labels, double-sided tapes, glue dots, note pads [1-3]. Differing from other types of adhesives showing permanent adhesion once being sticked, PSAs for removable application must be designed with a balance between flow and resistance to flow: the bond forms because the adhesive is soft enough to wet the adherend, and the adhesive is cohesive enough to resist the stress of the de-bonding process. Up to date, most of PSAs are derived from petroleum-based materials [4, 5] such as acrylic copolymers, styrene-butadiene rubber, styrene-butadiene-styrene block copolymers, showing negative impact on sustainable development [6-8]. Therefore, it is urgent to develop alternative products of petroleum-based PSA from renewable resources.
Among the different kinds of renewable raw materials, plant oils and their downstream derivatives have attracted great attention in both academic and industrial circles for fabricating lubricants, coatings, paints and bioplastics in recent years, and have been considered as one of the most biodegradable, least toxic, and cheapest alternatives to the petroleum-based chemicals [9-20]. Plant oils are triacylglycerols, of which the fatty acids are in majority composed, like palmitoleic acid (C16H30O2), oleic acid (C18H34O2), linoleic acid (C18H32O2), α-linolenic acid (C18H30O2), ricinoleic acid (C18H34O3), and so on. Among the rest, ricinoleic acid from the castor oil, formally called 12-hydroxy-9-cis-octadecenoic acid, is an unsaturated omega-9 fatty acid, which has been widely used in the preparation of various polymer materials (such as bio-based polyamide, polyurethane) [21-29] due to the coexistence of hydroxyl and carboxyl groups along the fatty chain. For instance, Chen and coworkers [21, 22] reported a series of bio-based re-processible poly(urethane urea) from ricinoleic acid. By incorporating isosorbide as stiff component to enhance network stiffness and reduce crosslink density, these castor oil derived poly(urethane urea) further exhibited simultaneous reinforcement and toughening [23]. Furthermore, the long aliphatic chains of ricinoleic acid could impart unique properties to the resulting polymeric materials such as elasticity, flexibility, hydrolytic stability, hydrophobicity and low glass transition temperatures, thus ricinoleic acid is undoubtedly suitable for producing PSAs.
Herein, we reported fully bio-based adhesives by curing the carboxyl-terminated polyricinoleate from ricinoleic acid and the epoxidized soybean oil (ESO). In order to achieve an optimized combination of resistance to shear and tacky character, the formulation ratio of the PSAs was comprehensively investigated. The adhesion/cohesion balance, as an important factor for an instant wetting of a surface by a light pressure and clear removal without residues on the applied surface, was studied in detail. Furthermore, the adhesive properties and the rheological performance of these PSAs were also investigated.
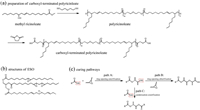
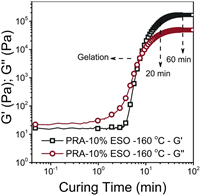
The carboxyl-terminated polyricinoleate (PRA) was first synthesized through melt polycondensation from methyl ricinoleate and diethylene glycol, as showing in Scheme 1a. Then, the obtained PRA was used to cure with ESO to obtain the fully bio-based PSA. During the curing process, the ring-opening of the epoxy groups with the -COOH groups took place [30, 31], producing new β-hydroxyl groups (Scheme 1c, path A). In addition, the epoxy groups of ESO were able to react with β-hydroxyesters (Scheme 1c, path B) and due to the high temperature during the curing process, -COOH groups possibly reacted with the -OH groups of β-hydroxyesters as well (Scheme 1c, path C). Fig. 1 showed the evolution of the storage modulus (G') and loss modulus (G") during isothermal curing at 160 ℃ for the adhesive formula PRA-10%ESO. At the beginning of the curing process, G' was lower than G", denoting a liquid-like response. Upon curing, both G' and G" increased, but the build-up rate of G' was higher than that of G". After the intersection (tanδ = 1) occurred after 9 min for PRA-10%ESO, G' kept increasing, whereas G" tended to reach a plateau corresponding to a more solid-like behavior with a high elastic part (G') compared to the viscous contribution (G").
|
Download:
|
| Scheme 1. (a) Two-step method for the preparation of the carboxyl-terminated polyricinoleate (PRA). (b) Chemical structure of epoxidized soybean oil (ESO). (c) Scheme of the three potential curing pathways: ring-opening esterification of epoxy with carboxyl-terminated polyesters (path A), etherification between oxirane and hydroxyl groups (path B), and condensation esterification between carboxyl and the resultant hydroxyl groups (path C). | |
|
Download:
|
| Fig. 1. Evolution of G' and G" with the curing reaction time at 160 ℃ for PRA-10%ESO. | |
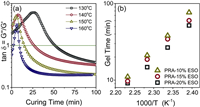
Fig. 2a displayed the evolution of the loss factor tanδ (tanδ = G"/G') as a function of time during the isothermal curing of PRA-10%ESO at different temperatures. With elevating temperature from 130 ℃ to 160 ℃, the time of tanδ = 1 point delayed. According to Flory's gelation theory [32], the chemical conversion of a thermosetting resin at the gel point was constant and not related to the reaction temperature and/or to the experimental conditions. As a result, the apparent activation energy Ea of the curing reaction could be estimated from the crossover time between the [tanδ = f(t)] and [tanδ=1] curves according to eq. 1, where C is a constant related with the pre-exponential factor, R is the gas constant, and T is the curing temperature:
|
Download:
|
| Fig. 2. (a) Loss factor tanδ versus reaction time for the adhesive PRA-10%ESO cured at different temperatures. (b) Arrhenius plot of the gelation time (as defined by the crossover between G' and G") as a function of the reciprocal temperature for PRA-α%ESO. | |
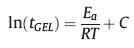
|
(1) |
Fig. 2b showed a plot of ln(tGEL) as a function of the reciprocal temperature for the system PRA-α%ESO from different combinations of base-polymers and cross-linkers. For each formulation, the good parallel fits indicated that the observed curing reaction was in good agreement with Eq. 1. The Ea values of PSAs were listed in Table 1. According to the data, the Ea values of the curing system with different ESO dosage were within the error range, indicating that the difficulty of curing reaction had no obvious correlation with the amount of ESO.
|
|
Table 1 Activation energy of PRA-α%ESO. |
Controlling over the sol/gel content was a key parameter for the preparation of PSA and, related to the ability of the material to dissipate energy through deformation. The gel content of general commercial PSAs was in the range of 40%~80% [33]. As shown in Fig. 3a, by adjusting the amount of crosslinking agent, the gel content of PRA-α%ESO could be controlled in the range of 5%~82%, with the increase in the dosage of ESO present a trend of increasing first and then decreasing, which met PSA preparation requirements.

|
Download:
|
| Fig. 3. (a) Gel content, (b) Tg and tensile force and (c) strain at break of the adhesive system PRA-α%ESO. | |
Commonly, the flexibility of the polymer chains was limited by the dense structure of the cross-linked polymers, which resulted in a higher Tg value compare to loose structure. As shown in Fig. 3b, PRA-15%ESO showed the highest gel content of 82%, correspondingly with the highest Tg value. The tensile experiments were useful to provide information on the large-strain (nonlinear) mechanical behavior of PSAs occurring during the de-bonding process (Fig. 3c). With the cross-linking density increased, the strength at break of PSAs increased. Among them, PRA-15%ESO showed the highest strength.
It was well established by many investigators that the performance of PSAs (e.g., peel, tack and shear) depends on the bulk viscoelastic behaviors of the adhesives [34]. Higher storage modulus (elastic modulus) in PSAs usually results in low adhesion, while a higher loss modulus (viscous modulus) in PSAs usually results in cohesion failure [35]. Therefore, an appropriate viscoelasticity must be achieved to optimize the balance between cohesion and adhesion strength for PSA applications. In Fig. 4a, PRA-15%ESO exhibited an elastic response (G' > G") over the whole frequency range; while PRA-10%ESO showed the loss modulus (G") slightly lower than G'. It was found that PRA-10%ESO samples were sticky to the touch (tacky).

|
Download:
|
| Fig. 4. The viscoelastic behavior of the testing PSAs. (a) G' and G" plots as function of testing frequency, (b) viscoelastic window and (c) creep resistance (strain recovery as a function of time). | |
According to Chang and Yang's works [36, 37], the response of the material during a bonding event correlated to a frequency of ~1 rad/s, while de-bonding under standard 180° peel testing conditions roughly corresponded to higher frequencies of ~435 rad/s. The viscoelastic windows of the PSAs were constructed for a better understanding of how the viscoelastic behavior at bonding and de-bonding frequencies affected the adhesives. As shown in Fig. 4b, the viscoelastic window of PRA-15%ESO laid within the high shear region (quadrant 2, top right quadrant). This quadrant corresponded to high modulus and high dissipation. In general the PSAs in this area possessed high Tg and highly cross-linked to achieve the high shear performance. The corresponding viscoelastic windows for PRA-10%ESO occupied the central region (overlapping part of the four quadrants), corresponding to medium modulus and medium dissipation; and the PSAs in this area showed liquid-like behavior to deform or flow in contact with a surface under light pressure. Consequently they had sufficient resistance to flow during a separation or de-bonding process [38]. PRA-8.75%ESO with loosely cross-linked network displayed crossovers between G' and G" (Fig. 4a), and were therefore characterized by viscous behaviors at low frequency. In this case, it was worthless to discuss the viscoelastic window of PRA-8.75%ESO.
In addition to adhesion, useful PSAs must also possess elasticity and resist flow under shear (known as resistance to creep) [4, 39] As illustrated in Fig. 4c, creep tests were performed for the system PRA-α%ESO with various cross-linking levels. PRA-15%ESO exhibited the lowest extent of strain after applying a stress for 10 min, which confirmed again the solid-like characteristic of this sample. PRA-10%ESO displayed a high percentage of recovery. The strain percentage of recovery after creep was related the density of the network. Therefore, PRA-8.75%ESO and PRA-20%ESO with looser networks displayed lower recovery than PRA-10%ESO did. Fig. 4c also confirmed that PRA-5%ESO was not cross-linked completely (very high creeping without any recovery).
Fig. 5a displayed the initial adhesion forces of the PSAs as a function of the network formulation. As expected, adhesion levels and debonding modes were influenced by the compounding ratio of the adhesives. The failure mode of PRA-8.75%ESO was cohesion dominant, sticky patches were observed on the steel substrate after peeling. Increasing the dosage of ESO to 15 wt% led to a considerable increase in tensile strength as aforementioned but decrease in cohesive strength. During peeling, PRA-10%ESO was deformed without leaving a sticky residue on the surfaces, and cavitation holes appeared on the adhesive's surface. PRA-15% ESO with the highest cross-linking density achieved the lowest peel strength (0.40 N/cm), suggesting the chain mobility and wettability of the PSA were sacrificed. In contrast, PRA-17.5%ESO and PRA-20%ESO exhibited higher adhesion force (1.56 N/cm and 2.10 N/cm, respectively) than PRA-15% ESO did, with a re-increase tendency.
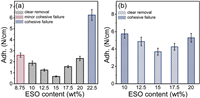
|
Download:
|
| Fig. 5. (a) Adhesion forces after 15 min dwell and (b) after 3 days at room temperature for PRA-α%ESO. | |
After a dwell period of 3 days at room temperature, the adhesion force of the PSAs increased (Fig. 5b). Notably, PRA-10%ESO showed a 300% adhesion increase on steel; and the de-bonding patterns changed from the interfacial failure between adhesive with steel to cohesive failure. As a result, adhesive residue could be observed on the steel substrate after peeling. Although PRA-12.5%ESO initially displayed slightly lower adhesion levels than PRA-17.5%ESO, its adhesion force dramatically increased (up to 280%) on steel after 3 days, resulting in PRA-12.5%ESO with adhesion of 4.4 N/cm, which was higher than PRA-17.5%ESO (230% increased) with adhesion of 4.2 N/cm.
In summary, a series of novel plant oil derived PSAs based on carboxyl-terminated polyricinoleate (PRA) and epoxiedized soybean oil (ESO) were obtained. Adhesive properties of PSA could be tailored by adjusting the component ratio, as well as the cross-linking level and the viscoelastic behavior thereof. At low cross-linking level, the PSAs behaved like a viscous liquid and did not possess enough cohesiveness to sustain the mechanical stress during peeling. The PSAs cross-linked at or near the optimal stoichiometric conditions displayed an adhesive (interfacial) failure between the substrate and the adhesive layer, which were associated with the lowest adhesion levels. The PSAs with the dosage amount of ESO ranging from 10 ~ 20 wt% were tacky and flexible, which exhibited 180° peel strength ranging from 0.4~2.3 N/cm; and could be easily removed without any residues on the adherend. The process for the preparation of the fully bio-based PSAs was environmentally friendly without using any organic solvent or other toxic chemical, herein showing the great potential as sustainable materials. Taking advantage of these features, this kind of bio-based removable PSA could bring more chances for versatile applications.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsFinancial supports by the National Natural Science Foundation of China (Nos. 51761135132 and 51822304) are sincerely acknowledged.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.06.015.
| [1] |
A. Beharaj, I. Ekladious, M.W. Grinstaff, Angew. Chem. Int. Ed. 58 (2019) 1407-1411. DOI:10.1002/anie.201811894 |
| [2] |
I. Benedek, M.M. Feldstein, Handbook of Pressure-Sensitive Adhesives and Products: Fundamentals of Pressure Sensitivity, Taylor & Francis Group, Boca Raton, 2009, pp. 1-2.
|
| [3] |
D. Satas, Handbook of Pressure Sensitive Adhesive Technology, 2nd ed., Van Nostrand Reinhold, New York, 1989, pp. 158-203.
|
| [4] |
R. Vendamme, N. Schüwer, W. Eevers, J. Appl. Polym. Sci. 131 (2014) 46169-46188. |
| [5] |
A. Gandini, Green Chem. 13 (2011) 1061-1083. DOI:10.1039/c0gc00789g |
| [6] |
M.R. Yates, C.Y. Barlow, Resour. Conserv. Recycl. 78 (2013) 54-66. DOI:10.1016/j.resconrec.2013.06.010 |
| [7] |
R.A. Gross, Kalra Bhanu, Science 297 (2002) 803-807. DOI:10.1126/science.297.5582.803 |
| [8] |
X. Zhang, M. Fevre, G.O. Jones, R.M. Waymouth, Chem. Rev. 118 (2018) 839-885. DOI:10.1021/acs.chemrev.7b00329 |
| [9] |
R.T. Zeng, Y. Wu, Y.D. Li, M. Wang, J.B. Zeng, Polym. Test. 57 (2017) 281-287. DOI:10.1016/j.polymertesting.2016.12.007 |
| [10] |
J.X. Wang, K.T. Chen, S.T. Huang, C.C. Chen, Chin. Chem. Lett. 22 (2011) 1363-1366. DOI:10.1016/j.cclet.2011.05.041 |
| [11] |
L.J. Sun, C. Yao, H.F. Zheng, J. Lin, Chin. Chem. Lett. 23 (2012) 919-922. DOI:10.1016/j.cclet.2012.05.030 |
| [12] |
M.M. Fan, J.J. Zhou, Q.J. Han, P.B. Zhang, Chin. Chem. Lett. 23 (2012) 1107-1110. DOI:10.1016/j.cclet.2012.07.009 |
| [13] |
T.H. Zhao, Y. Wu, Y.D. Li, M. Wang, J.B. Zeng, ACS Sustain. Chem. Eng. 5 (2017) 1938-1947. DOI:10.1021/acssuschemeng.6b02684 |
| [14] |
X.Y. Jian, Y. He, Y.D. Li, M. Wang, J.B. Zeng, Chem. Eng. J. 326 (2017) 875-885. DOI:10.1016/j.cej.2017.06.039 |
| [15] |
B.K. Ahn, S. Kraft, D. Wang, X.S. Sun, Biomacromolecules 12 (2011) 1839-1843. DOI:10.1021/bm200188u |
| [16] |
B.K. Ahn, J. Sung, X.S. Sun, J. Am. Oil Chem. Soc. 89 (2011) 909-915. |
| [17] |
A. Li, K. Li, RSC Adv. 5 (2015) 85264-85271. DOI:10.1039/C5RA15144A |
| [18] |
S.K. Sahoo, S. Mohanty, S.K. Nayak, RSC Adv. 5 (2015) 13674-13691. DOI:10.1039/C4RA11965G |
| [19] |
Z. Wang, L. Yuan, M.S. Ganewatta, et al., Macromol. Rapid Commun. 38 (2017) 1700009. DOI:10.1002/marc.201700009 |
| [20] |
T.H. Zhao, W.Q. Yuan, Y.D. Li, Y.X. Weng, J.B. Zeng, Macromolecules 51 (2018) 2027-2037. DOI:10.1021/acs.macromol.8b00103 |
| [21] |
J.H. Chen, D.D. Hu, Y.D. Li, et al., Polym. Test. 70 (2018) 174-179. DOI:10.1016/j.polymertesting.2018.07.004 |
| [22] |
J.H. Chen, D.D. Hu, Y.D. Li, et al., Polymer 143 (2018) 79-86. DOI:10.1016/j.polymer.2018.04.013 |
| [23] |
X.P. An, J.H. Chen, Y.D. Li, J. Zhu, J.B. Zeng, Sci. China Mater. 61 (2018) 993-1000. |
| [24] |
S. Miao, P. Wang, Z. Su, S. Zhang, Acta Biomater. 10 (2014) 1692-1704. DOI:10.1016/j.actbio.2013.08.040 |
| [25] |
Y. Xia, R.C. Larock, Green Chem. 12 (2010) 1893-1909. DOI:10.1039/c0gc00264j |
| [26] |
J. Hu, T. Rong, M.Z. Rong, M.Q. Zhang, Acta Polym. Sin. (2014) 276-285. |
| [27] |
J.H. Chen, W.Q. Yuan, Y.D. Li, Y.X. Weng, J.B. Zeng, ACS Sustain. Chem. Eng. 7 (2019) 15147-15153. DOI:10.1021/acssuschemeng.9b03956 |
| [28] |
T. Wang, L. Li, Y. Cao, Q. Wang, C. Guo, Ind. Crops Prod. 130 (2019) 562-570. DOI:10.1016/j.indcrop.2019.01.017 |
| [29] |
T. Wang, L. Li, Q. Wang, G. Xie, C. Guo, Ind. Crops Prod. 141 (2019) 111798. DOI:10.1016/j.indcrop.2019.111798 |
| [30] |
S.P. Bunker, R.P. Wool, J. Polym, Sci. A Polym. Chem. 40 (2002) 451-458. DOI:10.1002/pola.10130 |
| [31] |
S.N. Khot, J.J. Lascala, E. Can, et al., J. Appl. Polym. Sci. 82 (2001) 703-723. DOI:10.1002/app.1897 |
| [32] |
P.J. Flory, Principles of Polymer Chemistry, Cornell University Press, Ithaca, US, 1953.
|
| [33] |
S. Sohn, J. Adhes. Sci. Technol. 17 (2003) 889-901. DOI:10.1163/156856103321645220 |
| [34] |
C. Creton, G. Hu, F. Deplace, L. Morgret, K.R. Shull, Macromolecules 42 (2009) 7605-7615. DOI:10.1021/ma900821k |
| [35] |
C.A. Dahlquist, Adhes. Age 2 (1959) 25-29. |
| [36] |
E.P. Chang, J. Adhes. 34 (1991) 189-200. DOI:10.1080/00218469108026513 |
| [37] |
E.P. Chang, J. Adhes. 60 (1997) 233-248. DOI:10.1080/00218469708014421 |
| [38] |
A.E.O'Connor, N. Willenbacher, Int. J. Adhes. Adhes. 24 (2004) 335-346. DOI:10.1016/j.ijadhadh.2003.11.005 |
| [39] |
R. Vendamme, K. Olaerts, M. Gomes, et al., Biomacromolecules 13 (2012) 1933-1944. DOI:10.1021/bm300523e |
 2021, Vol. 32
2021, Vol. 32 


