b School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710119, China;
c Faculty of Physics, Bielefeld University, D-33501 Bielefeld, PO Box 100131, Germany
A hendecanuclear 3d-4f cluster [Cu6Gd5] with high molecular symmetry of D3h displays potential application in magnetic cooling, which thanks to the weak magnetic interactions between the component metal ions.
The explorations of polymetallic 3d-4f complexes have drawn considerable attention not only for their structural diversities, but also for their attractive luminescence, magnetic, and catalytic properties [1-3]. Up to now, impressive progress has been made to produce such species, such as [Cd24Ln8], [Ni21Gd20], [Ni12Gd36], [Co10Ln42], [Ni10Ln42], [Ni36Ln102], and [Ni64Ln96], which contain more than 30 metal centres [1-7]. Among these aggregations, owing to the large spin ground state and low anisotropy of the f7 Gd(III) ion, 3d-Gd clusters have been displayed to show great potential applications in low-temperature magnetic refrigeration [2].
Magnetic refrigeration, which works on the magneto-caloric effect (MCE), was among others studied by Warburg in 1881 [8, 9]. In recent years, due to the environmental pollution issues becoming more prominent, this environment friendly cooling technology has attracted much attention. To prepare magnetic coolers with large MCE, in addition to the factors mentioned above, several other ingredients also need to be considered such as the large metal/ligand mass ratio and the low spin excited state [2]. Furthermore, previous findings have shown that the ferro-magnetic exchange interactions can lead to large spin ground states and the weak exchange coupling constant can also ensure close-lying excited states, and subsequently help to enhance the MCE [2]. Controlling the sign of magnetic exchange is usually difficult, but we noticed the Cu(II)···Gd(III) magnetic interaction within 3d-4f clusters is frequently found to be weak ferromagnetic, owing to the orthogonality of the d- and f-orbitals and thus the electron transfer from the occupied 3d-orbital of Cu(II) ion to the empty 5d-orbital of Gd(III) ion [10]. To date, although there are several Cu-Gd magnetic refrigerants been reported, e.g., [Cu5Gd4], [Cu6Gd6], [Cu8Gd4], [Cu6Gd12], [Cu24Gd6], and [Cu36Gd24], in contrast to the reported Ni(II) and Co(II/III)-contained 3d-4f ones, the design of Cu-Gd clusters still remains a challenge [11]. The challenge stems from the different coordination behaviours of Cu(II) and Gd(III) ions [12]. One powerful strategy to construct such clusters relies on the use of amphipathic ligands, since 4f metal ions are more attracted to hard atoms like O, while 3d ions are likely to bind soft atoms such as N and S. Hence, we proposed a "mixed-ligand" strategy in our previous work, which exhibits great potential in shaping high-nuclearity 3d-4f clusters [3a, 4, 7].
Continuing our research, we herein selected 2-aminoisobutyric acid (Haib) which can bridge metal ions in multiple coordination modes with N and O atoms to synthesize Cu-Gd clusters, with the 2-hydroxypyridine (Hp) ligand used as an ancillary ligand. Haib was chosen because it was rarely employed to synthetize 3d-4f coordination clusters. After the simple hydrolysis reaction of Cu(II) and Gd(III) metal salts, Haib and Hp ligands in aqueous solution, a new family of isostructural 3d-4f clusters [Cu6Ln5(μ3−OH)9(C5H4ON)6(C4H8O2N)6(H2O)9]·(ClO4)6·(H2O)22 (Ln=Pr, 1; Nd, 2; Sm, 3; Eu, 4; Gd, 5) with high molecular symmetry of D3h (Fig. 1 and Fig. S1 in Supporting information), was successfully isolated. Notably, the [Cu6Ln5] clusters are rare examples of Haib-based 3d-4f clusters [13]. In addition, the magnetization data collected for complex 5 yield a magnetic entropy change (−ΔSm) of 19.6 J kg−1 K−1 at 3 K and 7 T which thanks to the weak magnetic interactions between the component metal ions.

|
Download:
|
| Fig. 1. (a) Ball-and-stick view of 5 with H atoms removed for clarity. (b) The arrangement of eleven metal ions in [Cu6Gd5] core. (c) The magnetic coupling schemes of the metal centers in [Cu6Gd5] core. Color codes: Gd, purple; Ni, green; N, blue; O, red; C, grey. | |
Single-crystal X-ray diffraction analyses reveal that complexes 1–5 are isostructural, thus only the structural details of 5 is described here (Tables S1 and S2 in Supporting Information). Complex 5 crystallizes in the cubic space group I-43d with the asymmetry unit containig one third of the cluster (Fig. S2 in Supporting information). The cationic [Cu6Ln5] core in 5 can be viewed as a trigonal bipyramid shape {Gd5} unit sandwiched by two equilateral {Cu3} triangles (Fig. 1b). Each of three equatorial Gd(III) ions (Gd3, Gd4 or Gd5) in {Gd5} unit connects with the adjacent two Gd(III) ions (Gd1 and Gd2) on the axis via three μ3−OH groups. The local symmetry of the former three Gd(III) ions is nearly D4d which compensated by three coordinated water molecules and two hydroxyl O atom from the ligand 2-hydroxylpyridine. The coordination sphere of each of the axial Gd(III) ions is completed by six μ3−OH groups and three carboxylate O atoms from Haib ligands. The Gd-O distances and the Gd(III)⋯Gd(III) separations in this core range from 2.32 Å to 2.54 Å and 3.96 Å–6.11 Å, respectively (Table S3 in Supporting information). Note that the Gd–O–Gd angles ranging from 97.1° to 111.6° average at 108.5° (< 110.9°), which usually causes antiferromagnetic exchange between Gd(III) ions [4, 14]. For two equilateral {Cu3} triangles, they linked to the central {Gd5} unit through six μ3−OH groups, six Haib and six Hp ligands to form a triangular prismatic core [Cu6Gd5] with the Cu(II)⋯Gd(III) distances ranging from 3.53 Å to 3.57 Å. Every Cu(II) ion in {Cu3} unit is penta-coordinated by one μ3−OH group, two carboxyl O atoms, one N atom from Haib and one N atom from Hp, forming a tetragonal pyramid geometry. The Haib ligand adopts the syn–anti bridging mode to connect adjacent Cu(II) ions to form the {Cu3} triangle. The Cu–O bond distances, Cu–N bond distances and Cu(II)⋯Cu(II) separations (range from 1.91 to 2.35, 1, 98 to 2.02, and 5.35 to 5.36 Å) are comparable to other copper-based coordination compounds [15].
The presence of the Gd(III) ions prompted us to investigate the magnetic properties of 5 in the context of developing molecule-based materials for magnetic cooling [2]. The direct-current (Dc) susceptibility data were collected on a polycrystalline powder sample in the temperature range of 2–300 K, as shown in Fig. 2a. The χmT value at room temperature is 41.7 cm3 K/mol, close to the expected value of 41.6 cm3 K/mol for five free Gd(III) ions (S = 7/2, g = 2) and six uncoupled Cu(II) ions (S = 1/2, g=2). Upon cooling, the χmT product preserve almost constant with slightly decrease to 41.2 cm3 K/mol at 30 K before a sudden drop to the minimum value of 18.1 cm3 K/mol at 2 K. The χm−1 versus T plot in the temperature range of 50–300 K can be nicely fitted into the Curie–Weiss equation, producing θ = −3.4 K (Fig. 2a, inset). The negative but small θ value indicates the presence of weak antiferromagnetic interactions or a combined action of spin–orbit coupling effects and weak ferromagnetic interactions between the metal centers.

|
Download:
|
| Fig. 2. (a) Temperature dependence plots of χmT for 5; Inset: The plots of χ–1 vs. T for 5. (b) Field-dependent magnetization plots for 5. | |
Meanwhile, the field-dependent molar magnetization of 5 was also measured at 2–10 K in the range of 0–7 T (Fig. 2b), which reveals that M increases upon increasing the magnetic field, almost saturation until 7 T (Mexp=40.5 Nβ, Msat=41 Nβ for five Gd(III) ions and six Cu(II) ions, where N is the Avogadro constant). The detailed investigation of inter-ion exchange coupling in polymetallic 3d–4f complexes has always been a challenge due to the large number of magnetic states [4, 16]. To further gain the magneto-structural correlation of 5, the magnetic interactions were modeled with self-written software [17], by using the Hamiltonian equation below:
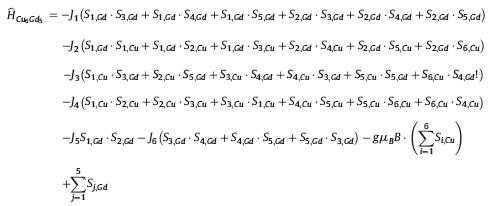
|
which uses the sign convention where J > 0 represents a ferromagnetic interaction, and we neglect the single ion magnetic anisotropy on the Gd(III) sites.
As shown in Fig. 1c, six exchange pathways were used to describe the system: J1 represents the interaction between axial Gd(III) ion and equatorial one (purple dash line); J2 for the interaction between axial Gd(III) ion and Cu(II) ion in {Cu3} triangle (solid red line); J3 for the interaction between equatorial Gd(III) ion and Cu(II) ion in {Cu3} triangle (dash red line); J4 for the interaction between Cu(II) ion in {Cu3} triangle (solid green line); J5 for the interaction between two axial Gd(III) ions (solid purple line); J6 for the interaction between Gd(III) ions in equatorial gadolinium triangle (fine solid purple line). By using the Hamiltonian described above, very good fits to the measured data in the range T = 2–65 K and 0–7 T were accomplished (Fig. 3), which gave the best-fit parameters shown in Fig. 3a. (legend). The fitting results indicate the exchange pathways via J1 and J5 would yield weak antiferromagnetic interactions. This result is good agree with the previous findings that if the Gd-O-Gd angle is smaller than 110.9°, interactions are anti-ferromagnetic; larger values correspond to ferromagnetic interactions [4, 14]. Whereas the coupling effect (via J2 exchange pathway) between the Cu(II) ions in {Cu3} triangle and the axial Gd(III) ion in {Gd5} unit is magnitude larger ferromagnetic interaction. This is again consistent with the previous reports [10, 18]. In addition, due to the Cu(II)⋯Cu(II) separation between the neighboring Cu(II) ions in {Cu3} triangle is relatively far (5.35–5.36 Å), the magnetic interactions via J4 exchange pathway can be omitted. The couplings via J6 exchange pathways are neglected not only because the large distance (6.11 Å) between the neighboring equatorial Gd(III) ions, but also because there is no bridging ligand/group between them. Summarizing, one can say that the best representation of the data under the assumptions of the Hamiltonian above, e.g., g=2.0 for all ions, is given by J1 = −0.16 K, J2 = 4 K, and a slightly antiferromagnetic J5. However, variations of the parameters of up to 20% still yield good results as can be seen in Fig. 3.

|
Download:
|
| Fig. 3. (a) Dc susceptibility data of 5 under 0.1 T (+ measured data) and calculation results. The numbers represent the respective values of J1, J2 and J5 (J3, J4 and J6 are omitted). (b) Field dependent magnetization data (+ measured data) of 5 and calculation results at 2 and 4 K. | |
The experimental magnetic entropy changes for 5 were then calculated by applying the Maxwell relation −ΔS(T)ΔB = ∫[∂M(T, B)/∂T]dB based on the magnetization data. The resulted maxima value at 3 K and 7 T for this complex is 19.6 J kg−1 K−1 (Fig. 4). This value is smaller than the maximum entropy value judged by the function of −ΔSm = nRln(2s + 1) = 15R, which corresponds to 34.1 J kg−1 K−1, but is medium among molecule based magnetic coolers [2]. The discrepancy may be attributed to the weak magnetic interactions between the neighboring metal centers. Moreover, the high symmetry of molecule can further reduce the anisotropy, which may contribute to the appreciable magneto-caloric effect.

|
Download:
|
| Fig. 4. Experimental −ΔSm values obtained from the magnetization data of 5. | |
In conclusion, a series of hendecanuclear isostructural [Cu6Ln5] clusters with high molecular symmetry of D3h was successfully synthesized via the simple hydrolysis reaction. Magnetic studies indicate that the [Cu6Gd5] displays significant magnetic entropy change at low temperature. The theoretical calculation confirmed the coexistence of both antiferromagnetic and ferromagnetic interactions between the metal centers. Further work on the Haib-based 3d-4f clusters is currently under investigation.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by Shenzhen Science and Technology Program (No. JCYJ20180306170859634), National Natural Science Foundation of China (Nos. 21773130, 21801202, 21871219, 21971203 and 21620102002), Shaanxi National Science Foundation (No. 2019JQ-016), China Postdoctoral Science Foundation (Nos. 2019T120891 and 2018M643615), Key Laboratory Construction Program of Xi'an Municipal Bureau of Science and Technology (No. 201805056ZD7CG40), Cyrus Chung Ying Tang Foundation and Fundamental Research Funds for Central Universities. This work was also supported by the Deutsche Forschungsgemeinschaft DFG (Nos. 314331397, SCHN615/23-1).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:
| [1] |
(a) X.P. Yang, D. Schipper, R.A. Jones, et al., J. Am. Chem. Soc. 135 (2013) 8468-8471; (b) X.P. Yang, D. Schipper, L.J. Zhang, et al., Nanoscale 6 (2014) 10569-10573. |
| [2] |
(a) Y.Z. Zheng, G.J. Zhou, Z. Zheng, R.E.P. Winpenny, Chem. Soc. Rev. 43 (2014) 1462-1475; (b) J.L. Liu, Y.C. Chen, F.S. Guo, M.L. Tong, Coord. Chem. Rev. 281 (2014) 26-49; (c) X.Y. Zheng, X.J. Kong, Z. Zheng, L.S. Long, L.S. Zheng, Acc. Chem. Res. 51 (2018) 517-525; (d) X.H. Miao, S.D. Han, S.J. Liu, X.H. Bu, Chin. Chem. Lett. 25 (2014) 829-834. |
| [3] |
(a) W.P. Chen, P.Q. Liao, P.B. Jin, et al., J. Am. Chem. Soc. 142 (2020) 4663-4670; (b) F. Evangelisti, R. Moré, F. Hodel, S. Luber, G.R. Patzke, J. Am. Chem. Soc. 137 (2015) 11076-11084; (c) K. Griffiths, P. Kumar, G.R. Akien, etal., Chem. Commun. 52 (2016) 7866-7869; (d) A. Baniodeh, N. Magnani, Y. Lan, et al., Npj Quantum Mater. 3 (2018) 10. |
| [4] |
W.P. Chen, J. Singleton, L. Qin, et al., Nat. Comm. 9 (2018) 2107. DOI:10.1038/s41467-018-04547-4 |
| [5] |
J.B. Peng, Q.C. Zhang, X.J. Kong, et al., Angew. Chem. Int. Ed. 50 (2011) 10649-10652. |
| [6] |
J.B. Peng, Q.C. Zhang, X.J. Kong, et al., J.Am. Chem. Soc. 134 (2012) 3314-3317. DOI:10.1021/ja209752z |
| [7] |
W.P. Chen, P.Q. Liao, Y. Yu, et al., Angew. Chem. Int. Ed. 55 (2016) 9375-9379. DOI:10.1002/anie.201603907 |
| [8] |
(a) E. Warburg, Ann. Phys. Chem. 13 (1881) 141-164; (b) A. Smith, Eur. Phys. J. H 38 (2013) 507-517. |
| [9] |
(a) B.F. Yu, Q. Gao, B. Zhang, X.M. Meng, X.Z. Chen, Int. J. Refrig. 26 (2003) 622-636; (b) V. Pecharsky, K.A.J. Gschneidner, Int. J. Refrig. 29 (2006) 1239-1249; (c) K.A.J. Gschneidner, V. Pecharsky, Int. J. Refrig. 31 (2008) 945-961. |
| [10] |
(a) D. Dermitzaki, G. Lorusso, C.P. Raptopoulou, et al., Inorg. Chem. 52 (2013) 10235-10237; (b) J.J. Zhang, S.M. Hu, S.C. Xiang, et al., Inorg. Chem. 45 (2006) 7173-7181; (c) J. Wu, L. Zhao, L. Zhang, et al., Angew. Chem. Int. Ed. 55 (2016) 15574-15578; (d) V. Baskar, K. Gopal, M. Helliwell, et al., Dalton Trans. 39 (2010) 4747-4750; (e) M. Andruh, I. Ramade, E. Codjovi, et al., J. Am. Chem. Soc. 115 (1993) 1822-1829. |
| [11] |
(a) S.K. Langley, N.F. Chilton, B. Moubaraki, et al., Chem. Sci. 2 (2011) 1166-1169; (b) A.S. Dinca, A. Ghirri, A.M. Madalan, M. Affronte, M. Andruh, Inorg. Chem. 51 (2012) 3935-3937; (c) T.N. Hooper, J. Schnack, S. Piligkos, M. Evangelisti, E.K. Brechin, Angew. Chem. Int. Ed. 51 (2012) 4633-4636; (d) D.I. Alexandropoulos, K.M. Poole, L. Cunha-Silva, et al., Chem. Commun. 53 (2017) 4266-4269; (e) G. Xiong, H. Xu, J.Z. Cui, Q.L. Wang, B. Zhao, Dalton Trans. 43 (2014) 5639-5642; (f) J.D. Leng, J.L. Liu, M.L. Tong, Chem. Commun. 48 (2012) 5286-5288. |
| [12] |
X.Y. Zheng, X.J. Kong, L.S. Long, Lanthanide hydroxide cluster complexes via ligand-controlled hydrolysis of the lanthanide ions, in: Z. Zheng (Ed. ), Recent Development in Clusters of Rare Earths and Actinides: Chemistry and Materials, Springer, Berlin, 2017, pp. 51-96.
|
| [13] |
(a) M. Orfanoudaki, I. Tamiolakis, M. Siczek, et al., Dalton Trans. 40 (2011) 4793-4796; (b) G.J. Sopasis, A.B. Canaj, A. Philippidis, et al., Inorg. Chem. 51 (2012) 5911-5918; (c) G.J. Sopasis, M. Orfanoudaki, P. Zarmpas, et al., Inorg. Chem. 51 (2012) 1170-1179. |
| [14] |
S.C. Xiang, S.M. Hu, T.L. Sheng, et al., J. Am. Chem. Soc. 129 (2007) 15144-15146. DOI:10.1021/ja0760832 |
| [15] |
(a) M.B. Nasr, K. Kaabi, M. Zeller, et al., Chin. Chem. Lett. 27 (2016) 896-900; (b) D. Tian, X.J. Liu, R.Y. Chen, Y.H. Zhang, Chin. Chem. Lett. 26 (2015) 499-503. |
| [16] |
(a) S.G. Tabrizi, A.V. Arbuznikov, M. Kaupp, J. Phys. Chem. A 120 (2016) 6864-6879; (b) V.V. Mazurenko, Y.O. Kvashnin, F. Jin, et al., Phys. Rev. B 89 (2014) 214422; (c) J. Zhang, L. He, H. Cao, F. Wang, P. Zhang, J. Chem. Phys. 128 (2008) 154711. |
| [17] |
(a) J. Schnack, O. Wendland, Eur. Phys. J. B 78 (2010) 535-541; (b) J. Schnack, C. Heesing, Eur. Phys. J. B 86 (2013) 46. |
| [18] |
(a) Y. Wang, W.S. Wu, M.L. Huang, Chin. Chem. Lett. 27 (2016) 423-427; (b) C.W. Yan, Y.T. Li, D.Z. Liao, Chin. Chem. Lett. 7 (1996) 681-684. |
 2021, Vol. 32
2021, Vol. 32 

