b College of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University, Ji'nan 250014, China
With the rapid development of biomedical science, more and more information demonstrates that microRNA (miRNA) has been regarded as a promising biomarker for cancer early screening and therapy due to its higher sensibility than body symptom [1, 2]. However, sensitive and selective detection of miRNA is always challenged by small size, high similarity, and low expression level [3, 4]. Motivated by this, flourishing studies have been made to pursue novel sensing strategies [6, 7]. Nevertheless, some inherent problems still exist and hamper the early and reliable diagnosis of cancers. For example, most biosensors devoted themselves to analysis of a single miRNA and thus easily made a misdiagnosis [5, 6]. Another intractable obstacle is that these biosensors require bio-enzymes to obtain high sensitivity, whereas the activity of which is easily influenced by environmental factors. Also, bio-enzyme is expensive [7, 8]. The both issues impel us to rethink enzyme-free yet highly sensitive strategy for multiple miRNAs simultaneous biosensing.
To get accurate identification of cancers, recently, fluorescent and electrochemical biosensors have been invented for simultaneous detection of multiple biomarkers [5, 9-12]. However, they suffered from an intrinsically isogenetic drawback of input energy and output signal, which causes comparatively high background, further decreasing sensitivity and accuracy. Different from them, photoelectrochemical (PEC) biosensors not only possess the advantages of both fluorescent and electrochemical techniques, but also achieve the complete separation of excitation light and PEC current [13-15]. Integrating a photoactive material with a recognizable molecule enables PEC biosensor selective identification of target analyte, providing a probability to effect multiple miRNAs simultaneous biosensing. Unfortunately, the study for multiple miRNAs simultaneous biosensing has been reported rarely due to the high requirement of wavelength-resolution photoactive materials.
Featured with high photochemistry performance, quantum dots (QDs) widely function as signal sources in development of fluorescent [16, 17] and PEC biosensors [18, 19]. To endow them the capability of multiple assay, QDs were applied as one of signal sources for multiple configurations fabrication based on fluorescence technique. However, to our best knowledge, there was no work reported with the involvement of PEC technique. Meanwhile, it is impracticable for QDs alone to develop multiple miRNAs biosensor. In addition to QDs, organic dyes have also been proven to be high-performance photoactive materials. Among them, methylene blue (MB) was widely applied in development of PEC biosensors due to low cost, mature modification technique with biomolecules, and unique intercalation/diffusion characteristic [20, 21]. Further, it was reported that MB has absorption range from 550 nm to 700 nm, which is different from QDs. From this context, MB and QDs can convert different wavelength of lights into currents, and their excitation wavelengths don't interfere with each other [20, 22]. Thus, MB and QDs can be applied as dual signal sources to develop a PEC biosensor for dual miRNAs simultaneous biosensing, whereas, which has not been investigated yet.
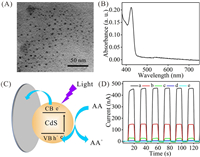
Herein, we propose a QDs/MB-based PEC biosensor for simultaneous determination of dual miRNAs based on strand displaced amplification (SDA). Compared with bioenzymes, SDA not only reduces the background and lowers the detection cost, but also avoids the sophisticated design of deoxyribonucleic acid (DNA) sequences [23]. In this study, CdS QDs were used as one of the photoactive substances and synthesized through hydrothermal reaction between thioacetamide and CdCl2 by using 3-mercaptopropionic acid (MPA) as a stabilizing agent [20]. Transmission electron microscopy (TEM) and zeta potential characterizations demonstrated that CdS QDs displayed a uniform globular structure with diameter of 4.0 nm (Fig. 1A), and a negative zeta value at −21.9 mV, which was ascribed to MPA anchored on CdS QDs' surface (Fig. S1 in Supporting information) and implied the exceptional water stability. Further, CdS QDs' UV–vis spectrophotometer (UV–vis) property was discussed. As manifested in Fig. 1B, CdS QDs show a peak at 423 nm with an absorbance at 0.21 a.u. With the wavelength increasing, the absorbance gradually reduced. When the wavelength was larger than 450 nm, there was no absorbance observed. In this context, it can be deduced that CdS QDs output high PEC current under < 450 nm light excitation accompanied by weak current under > 450 nm light irradiation. To validate this, PEC behavior of CdS QDs was investigated by using ascorbic acid (AA) as sacrificial electron donors, and the illustration was manifested in Fig. 1C. Under light illumination, CdS QDs changed from ground state to excitation state, and then the photoelectrons diffused to indium tin oxide (ITO) electrode and excited holes transferred into system to react with AA. Due to the separation of holes and electrons, the currents produced. As shown in Fig. 1D, under 430 nm light (which is proximate to 423 nm light in our laboratory) irradiation, CdS/ITO presented a current at 446 nA, and the current varied slightly under six on-off periods, justifying their high stability. Increasing the lights' wavelength into 440 nm, 450 nm, and 500 nm, PEC current respectively reduced. Besides, PEC current was only 3.5 nA under 627 nm light excitation, which is much lower than that of CdS/ITO under 430 nm light irradiation. In view of this, if there is a photoactive matter that can convert 627 nm light into current without any interference from 430 nm light, it was possible to develop a PEC biosensor for dual analytes simultaneous detection.
|
Download:
|
| Fig. 1. (A) TEM image of CdS QDs. (B) UV–vis spectrum of CdS QDs in solution. (C) Diagram of CdS QDs coating on ITO for converting light to current. (D) PEC currents of CdS/ITO under different wavelength of lights irradiation: (a) 430 nm, (b) 440 nm, (c) 450 nm, (d) 500 nm, and (e) 627 nm. | |
The UV–vis and PEC properties of MB were subsequently studied. Fig. S2A (Supporting information) displayed that the maximum peak was at 663 nm, and there was no absorption when the wavelength was < 540 nm. In addition, MB also has high absorbance at 627 nm (which is proximate to 663 nm in our laboratory), which is much larger than that at 430 nm. As expected, MB displayed a 366 nA current under 627 nm light excitation with strong stability (Fig. S2B in Supporting information). The PEC reaction was inferred as follows: MB adsorbs the energy of light to become into MB*, which produced the photoelectrons and excited holes, transferring to ITO electrode and reaction solution, respectively. Under other lights irradiation (590, 580, 500 nm), PEC current gradually decreased with wavelength reducing. When the wavelength decreased to 430 nm, only a 2.57 nA current was recorded. The above experimental results show that the excitation wavelengths of CdS QDs and MB do not interfere with each other for simultaneous PEC detection. In consideration of the complete separation of excitation wavelengths, we believe that it is feasible to apply CdS QDs and MB to develop a PEC biosensor for dual miRNAs simultaneous biosensing.
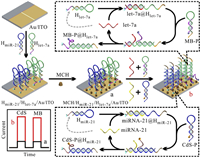
To realize miRNA-21 and let-7a simultaneous biosensing, DNA functionalized CdS QD (CdS-P) was prepared through amide reaction, and was characterized in Figs. S3 and S4 (Supporting information). By employing CdS-P and DNA functionalized MB (MB-P) as two wavelength-resolution signal sources, we tried our best to develop a PEC biosensor for highly sensitive and simultaneous detection of miRNA-21 and let-7a based on SDA, and the working mechanism was illustrated in Scheme 1. First, ITO electrode was electrochemically treated in HAuCl4 solution to obtain Au/ITO electrode. Au/ITO electrode was divided into WA1 and WA2, which were applied to linking with HmiR-21 and Hlet-7a through Au-S bond, respectively. Subsequently, mercaptohexanol (MCH) was employed to assemble on electrode to obtain MCH/Hlet-7a/HmiR-21/Au/ITO. In the absence of target miRNAs, hairpin configurations of HmiR-21 and Hlet-7a did not vary, resulting in no immobilization of CdS-P and MB-P. Both PEC currents were ultralow. Once only miRNA-21 or let-7a was added, it recognized and hybridized with HmiR-21 or Hlet-7a to form miRNA-21@HmiR-21 or let-7a@Hlet-7a complex via toehold-mediated strand displacement reaction. Meanwhile, the formed miRNA-21@HmiR-21 or let-7a@Hlet-7a complex could acted as a toehold to hybridize with CdS-P or MB-P, leading to a successful immobilization of CdS QDs or MB. Along with the strand displacement reaction, miRNA-21 or let-7a was released, and hybridized with unreacted HmiR-21 or Hlet-7a to initiate the next strand displacement reaction. Obviously, whether miRNA-21 or let-7a can realize the immobilization of abundant photoactive materials. But what we need to pay attention that only CdS-P or MB-P could be immobilized, thereby resulting in only one higher current. When miRNA-21 and let-7a were added, both CdS-P and MB-P were simultaneously immobilized, contributing to both high PEC currents. The increasing PEC currents of CdS QDs and MB upon the addition of miRNA-21 and let-7a showed that our PEC biosensor for dual miRNAs enzyme-free, highly sensitive and simultaneous biosensing is feasible.
|
Download:
|
| Scheme 1. Diagram illustration of working principle of CdS QDs/MB-based PEC biosensor for simultaneous detection of miRNA-21 and let-7a. | |
To validate the fabrication of CdS QDs/MB-based PEC biosensor, several electrochemical measurements were conducted. As shown in Fig. S5 (Supporting information), the current under −0.2 V gradually decreased along with the time increasing, and leveled off when the time was extended to 20 s, confirming the effective deposition of Au on ITO surface. Subsequently, electrochemical impedance spectroscopy (EIS) was performed (Fig. S6A in Supporting information). Au/ITO demonstrated a 78 Ω electron transfer resistance (Ret). When HmiR-21 was immobilized, increased due to the fact that immobilized HmiR-21 enhances the electrostatic repulsion of electrode toward [K3Fe(CN)6]/[K4Fe(CN)6]. In this context, when Hlet-7a and MCH were successively immobilized, Ret increased to 109 Ω and 148 Ω, respectively. Upon the addition of miRNA-21 and let-7a, Ret further increased, implying the efficient recognition and hybridization of MCH/Hlet-7a/HmiR-21/Au/ITO by target miRNAs. When CdS-P and MB-P were successively introduced, abundant CdS-P and MB-P were immobilized based on SDA and resulted in higher Ret, respectively. The gradually increased Ret in the presence of miRNA-21 and let-7a suggested that CdS QDs/MB-based PEC biosensor was successfully developed. Moreover, the fabrication process can also be verified by cyclic voltammetry (CV) technique (Fig. S6B in Supporting information).
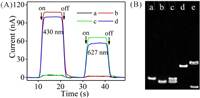
To confirm the feasibility for target miRNAs simultaneous biosensing, PEC curves under different conditions were recorded (Fig. 2A). If no target existed, PEC currents were only 3.7 nA and 1.9 nA. With only miRNA-21 being added, PEC current under 430 nm light excitation elevated to 108 nA accompanied by a weak current under 627 nm light excitation. In addition, if only let-7a was introduced, PEC current under 627 nm light irradiation increased to 66 nA accompanied by a weak current under 430 nm light excitation. This is because that efficient immobilization of CdS QDs (MB) is relied on target-switched SDA. Consequently, when both of them were added, PEC currents under 430 nm and 627 nm lights irradiation elevated to 99.7 nA and 56.5 nA, respectively. Both of the increased PEC currents indicated that CdS QDs/MB-based PEC biosensor can simultaneously detect miRNA-21 and let-7a. Furthermore, gel electrophoresis analysis was employed to validate the recognition of HmiR-21 (Hlet-7a) by target miRNA and competitive hybridization of HmiR-21 (Hlet-7a) with CdS-P (MB-P) (Fig. 2B and Fig. S7 in Supporting information).
|
Download:
|
| Fig. 2. (A) PEC curves of CdS QDs/MB-based PEC biosensor under different conditions: (a) no targets, (b) 10 pmol/L miRNA-21, (c) 10 pmol/L let-7a, (d) 10 pmol/L miRNA-21 + 10 pmol/L let-7a. (B) PAGE images of (a) HmiR-21′, (b) CdS-P', (c) HmiR-21′ + CdS-P', (d) HmiR-21′ + miRNA-21, (e) HmiR-21′ + CdS-P' + miRNA-21. | |
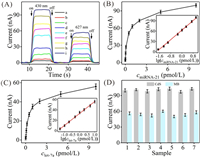
Prior to evaluate the ability of CdS QDs/MB-based PEC biosensor for miRNA-21 and let-7a simultaneous biosensing, HmiR-21 concentration, Hlet-7a concentration, CdS-P concentration, MB-P concentration, reaction time, and area ratio of WA1/WA2 that played significant influences in immobilization of photoactive materials were discussed (Figs. S8-S10 in Supporting information). After the optimization (1 μmol/L, 2 μmol/L, 1 μmol/L, 2 μmol/L, 120 min, and 1/2), we challenged CdS QDs/MB-based PEC biosensor by probing miRNA-21 and let-7a with varying amounts to assess its sensing ability. As displayed in Fig. 3A, PEC currents augmented with both of the miRNAs amounts elevating, which were due to working mechanism that more miRNAs initiated more SDA, thereby increasing the immobilization amounts of CdS QDs and MB. To precisely appraise the responsive sensitivity, working curves were made by using ICdS and IMB as vertical coordinates, miRNA-21 concentration (CmiRNA-21) and let-7a concentration (Clet-7a) as horizontal coordinates, respectively. The curves in Fig. 3B showed that ICdS maintained linearly with logarithm of miRNA-21 concentrations from 0.02 pmol/L to 10 pmol/L. The equation was calculated to be ICdS = 34.7142lgCmiRNA-21 + 63.0951 with coefficient of 0.9971 and detection limit of 6.6 fmol/L based on three sigma rule (3σ). For let-7a, the information was displayed in Fig. 3C, giving the equation of IMB = 22.9681lgClet-7a + 33.2531, coefficient of 0.9977 and detection limit of 15.4 fmol/L based on 3σ. The comparison of our biosensor and other sensors was summed up in Table S2 (Supporting information), demonstrating that the detection limit was comparable or lower than that of previously reported fluorescent/electrochemical biosensors dedicated to dual miRNAs biosensing whether or not they applied bioenzymes to amplify the signal.
|
Download:
|
| Fig. 3. (A) PEC curves of CdS QDs/MB-based PEC biosensor corresponding to miRNA-21 and let-7a with different concentrations: (a) 0 + 0, (b) 0.02 + 0.05 pmol/L, (c) 0.05 + 0.1 pmol/L, (d) 0.1 + 0.2 pmol/L, (e) 0.5 + 0.5 pmol/L, (f) 1 + 1 pmol/L, (g) 2 + 2 pmol/L, (h) 5 + 5 pmol/L, (i) 10 + 10 pmol/L. (B) PEC currents versus miRNA-21 concentrations. Inset shows the linear curve between ICdS and lgCmiRNA-21. (C) PEC currents versus let-7a concentrations. Inset shows the linear curve between IMB and lgClet-7a. (D) PEC currents of seven freshly prepared MCH/Hlet-7a/HmiR-21/Au/ITO electrodes in the presence of target miRNAs. | |
As is well known that selectivity acts an important role in assuring diagnosis accuracy, and thus, was studied through applying miRNA-155, miRNA-16, let-7b, let-7d, alanine aminotransferase (ALT), L-serine, taurine, and glucose as interferences. The information in Fig. S11 (Supporting information) demonstrated that when only target miRNAs were present, CdS QDs/MB-based PEC biosensor displayed two high PEC currents. These interferences made little dedication to change in ICdS and IMB. Thus, CdS QDs/MB-based PEC biosensor enjoys exceptional specificity for differencing miRNA-21 and let-7a against other interferences. Besides selectivity, repeatability is also a momentous criterion for a biosensor, and thus was discussed (Fig. 3D). Seven freshly prepared MCH/Hlet-7a/HmiR-21/Au/ITO electrodes were utilized to probe 10 pmol/L miRNA-21 and 10 pmol/L let-7a. For miRNA-21 and let-7a, the maximum current changes were 3.3 and 6.0 nA, which are small, justifying the excellent repeatability.
Breast cancer is the second most common cancer in women. It is of highly important to find effective strategy to achieve breast cancer early accurate diagnosis before it has spread. In this work, CdS QDs/MB-based PEC biosensor was employed to determine miRNA-21 and let-7a in serum from breast cancer patient, and the result was compared with qRT-PCR. Prior to analysis, the serum was diluted by PB buffer. The levels of miRNA-21 and let-7a were calculated to be 24.3 and 21.2 nmol/L by our biosensor, respectively, which were in good agreement with that gotten from qRT-PCR (Fig. S12 in Supporting information) and corresponded well to previously reported value [5]. Therefore, CdS QDs/MB-based PEC biosensor provides a significant potential for clinical determination of cancers.
In summary, a novel CdS QDs/MB-based PEC biosensor based on SDA for highly sensitive and simultaneous determination of miRNA-21 and let-7a has been developed. CdS QDs and MB are applied as photoactive substances, from which PEC currents generated under different lights irradiation. Profiting from target miRNAs-switched SDA, more CdS QDs and MB were immobilized on electrode, further enhancing PEC currents. Consequently, CdS QDs/MB-based PEC biosensor exhibited ultrahigh sensitivity toward miRNA-21 and let-7a with detection limits of 6.6 fmol/L and 15.4 fmol/L, respectively. This PEC biosensor was further employed to simultaneously determine miRNA-21 and let-7a in breast cancer patient's serum. This work not only exhibited a significant potential for high-performance PEC biosensor development, but also provided a promising tool for early and reliable determination of miRNA-related diseases.
Declaration of competing interestThere are no conflicts to declare.
AcknowledgmentsThis work was funded by the National Natural Science Foundation of China (Nos. 21605093 and 21775082), the Shandong Province Higher Educational Program for Young Innovation Talents, the Special Foundation for Distinguished Taishan Scholar of Shandong Province (No. ts201511052), and the Major Program of Shandong Province Natural Science Foundation (No. ZR2018ZC0127).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.05.041.
| [1] |
Y.Q. Xia, R.L. Zhang, Z.L. Wang, J. Tian, X.Y. Chen, Chem. Soc. Rev. 46 (2017) 2824-2843. DOI:10.1039/C6CS00675B |
| [2] |
B. Wu, C. Eliscovich, Y.J. Yoon, R.H. Singer, Science 352 (2016) 1430-1435. DOI:10.1126/science.aaf1084 |
| [3] |
R.J. Deng, L.H. Tang, Q.Q. Tian, et al., Angew. Chem. Int. Ed. 126 (2014) 2421-2425. DOI:10.1002/ange.201309388 |
| [4] |
X. Miao, Z. Cheng, H. Ma, et al., Anal. Chem. 90 (2018) 1098-1103. DOI:10.1021/acs.analchem.7b01991 |
| [5] |
J.F. Chang, X. Wang, J. Wang, H.Y. Li, F. Li, Anal. Chem. 91 (2019) 3604-3610. DOI:10.1021/acs.analchem.8b05599 |
| [6] |
W. Pan, B. Liu, X.N. Gao, et al., Nanoscale 10 (2018) 14264-14271. DOI:10.1039/C8NR04106G |
| [7] |
Y.H. Yuan, Y.D. Wu, B.Z. Chi, et al., Biosens. Bioelectron. 97 (2017) 325-331. DOI:10.1016/j.bios.2017.06.022 |
| [8] |
A. Vázquez-Guardado, S. Barkam, M. Peppler, et al., Nano Lett. 19 (2019) 449-454. DOI:10.1021/acs.nanolett.8b04253 |
| [9] |
J.R. Wang, Z.X. Lu, H.L. Tang, et al., Anal. Chem. 89 (2017) 10834-10840. DOI:10.1021/acs.analchem.7b02342 |
| [10] |
S. Wu, C. Li, H. Shi, Y. Huang, G.X. Li, Anal. Chem. 90 (2018) 9929-9935. DOI:10.1021/acs.analchem.8b02127 |
| [11] |
W.J. Wang, Y.L. Cai, B.C. Li, et al., Chin. Chem. Lett. 29 (2018) 111-114. DOI:10.1016/j.cclet.2017.05.009 |
| [12] |
Q. Zhou, G.H. Li, K.Y. Chen, et al., Anal. Chem. 92 (2020) 983-990. DOI:10.1021/acs.analchem.9b03915 |
| [13] |
Y. Zhang, N. Hao, Z. Zhou, et al., Chem. Commun. 53 (2017) 5810-5813. DOI:10.1039/C7CC01582H |
| [14] |
Q. Zhou, H. Xue, Y.Y. Zhang, et al., ACS Sens. 3 (2018) 1385-1391. DOI:10.1021/acssensors.8b00307 |
| [15] |
Y.Q. Lv, Z.X. Zhou, Y.F. Shen, et al., ACS Sens. 3 (2018) 1362-1367. DOI:10.1021/acssensors.8b00292 |
| [16] |
S.W. Yang, J. Sun, P. He, et al., Chem. Mater. 27 (2015) 2004-2011. DOI:10.1021/acs.chemmater.5b00112 |
| [17] |
Q. Liu, D.B. Zhu, M.L. Guo, Y. Yu, Y.J. Cao, Chin. Chem. Lett. 30 (2019) 1639-1642. DOI:10.1016/j.cclet.2019.05.058 |
| [18] |
N. Zhang, L. Zhang, Y.F. Ruan, et al., Biosens. Bioelectron. 94 (2017) 207-218. DOI:10.1016/j.bios.2017.03.011 |
| [19] |
G.N. Cai, Z.Z. Yu, R.R. Ren, D.P. Tang, ACS Sens. 3 (2018) 632-639. DOI:10.1021/acssensors.7b00899 |
| [20] |
Q. Hao, X.N. Shan, J.P. Lei, et al., Chem. Sci. 7 (2016) 774-780. DOI:10.1039/C5SC03336E |
| [21] |
T. Hou, N.N. Xu, W.X. Wang, L. Ge, F. Li, Anal. Chem. 90 (2018) 9591-9597. DOI:10.1021/acs.analchem.8b02523 |
| [22] |
Y.N. Zheng, W.B. Liang, C.Y. Xiong, et al., Anal. Chem. 89 (2017) 9445-9451. DOI:10.1021/acs.analchem.7b02270 |
| [23] |
F.C. Simmel, B. Yurke, H.R. Singh, Chem. Rev. 119 (2019) 6326-6369. |
 2021, Vol. 32
2021, Vol. 32 

