b College of Chemical Engineering, China University of Mining and Technology, Xuzhou 221116, China;
c University of Chinese Academy of Sciences, Beijing 100049, China
Organic light-emitting diodes (OLEDs) have attracted much attention in academic and industrial communities owing to their low energy cost, high-quality color, light weight, rapid response and flexibility [1-4]. However, the traditional fluorescent emitters for OLEDs still require improvement in terms of their devices cannot exceed the theoretical maximum exciton utilization of 25%, according to the principle of conservation of spin [5-7]. Although phosphorescent OLEDs can fully utilize electrogenerated singlet and triplet excitons to provide high electroluminescence (EL) efficiency by utilizing precious-metal-based phosphorescent materials to increase spin-orbit coupling of singlet (S1) state and triplet (T1) state [8-11]. These phosphorescent materials are expensive for containing transition metals such as iridium and platinum [12-14], which can raise the manufacturing cost and limit their practical applications [15]. As the promising third-generation organic luminescent material after the conventional fluorescent and phosphorescent materials, purely organic materials with thermally activated delayed fluorescence (TADF) have attracted considerable attention due to their high exciton utilization efficiency [16-20].
TADF emitters for OLEDs have made great progress so far [21-25]. However, the development of high-performance OLED devices generally needed guest-host doping technique to alleviate concentration-caused emission quenching and exciton annihilation processes [26, 27]. The doping technique requires complicated control of the doping concentration [28] and the reproducibility of OLED device is cumbersome as well [29-32]. Owing to their rigid structures and special properties, heterocyclic compounds have been widely utilized as acceptors for TADF materials, which included pyrimidine [33], heptazine [34], quinoxzline [35], phenanthroline [36], triazine [37, 38], pyrazine [39, 40], dibenzo[a, j]phenazine [41] and naphthyridine [42]. Among different kinds of heterocycles, quinoline derivatives stand out as important molecules with a wide range of interesting pharmacological activity and unique physicochemical property. However, there have been no reports quinoline-based TADF emitters and their applications in OLEDs so far. Herein, we report three emitters Fene, Fens and Yad with quinoline as a new electron acceptor. These emitters have strong intramolecular interactions, which can cause stable excited state configuration and restrict aggregation-caused quenching (ACQ) effect. Consequently, the emitters exhibited aggregation-induced emission (AIE) and aggregation-induced delayed fluorescence (AIDF) properties, and highly efficient non-doped OLEDs based on Fene, Fens and Yad achieved high maximum EQEs of 14.9%, 13.1% and 17.4%, respectively.
The synthetic routes of Fene, Fens and Yad were shown in Scheme 1. According to the literature procedure [43], 8-bromo-2-chloroquinoline was prepared. Then, compounds Fene, Fens and Yad were conveniently synthesized in good yields by palladium-catalyzed cross-coupling reactions of 8-bromo-2-chloroquinoline with 9, 9-dimethyl-acridan, phenothiazine and phenoxazine, respectively. The target compounds were purified via column chromatography and temperature-gradient sublimation with high vacuum conditions. Their structures were confirmed by 1H NMR, 13C NMR, HRMS spectra and single-crystal X-ray structures.

|
Download:
|
| 1. Synthesis of emitters Fene, Fens and Yad. | |
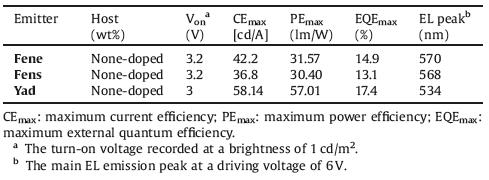
Single crystals of emitters Fene, Fens and Yad suitable for X-ray diffraction analyses were obtained from their solution in CH2Cl2/petroleum ether. As depicted in Fig. 1, the torsion angles of Fene between the two phenoxazine units and the quinoline moiety are 106.31° and 30.98°, respectively. The twisted molecular structure could be helpful for separating its highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO), resulting in small ΔEST. Moreover, the twisted conformation of Fene could also weaken the intermolecular interactions, which would greatly suppress concentration-caused emission quenching. Similarly, it was found that the torsion angles between the two phenothiazine units and the quinoline moiety in Fens are 159.63° and 72.86°, and the torsion angles between the two acridine units and the quinoline moiety in Yad are 171.84° and 75.51°. Compound Fene existed strong multiple intramolecular interactions with the distances from 2.83 Å to 3.21Å between the donor and the acceptor. Similarly, it was also found that there existed the multiple interactions between the donor and the acceptor of Fens with the distances from 2.95 Å to 3.20Å, and the multiple π-π interactions between the donor and the acceptor of Yad with the distances from 2.87 Å to 3.24Å. These multiple interactions could be helpful to rigidify the molecular structures and greatly suppress the exciton annihilation.
|
Download:
|
| Fig. 1. The torsion angles of (a) Fene, (b) Fens and (c) Yad. | |
In order to predict the performance of these luminogens, the spatial distributions of the highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) of Fene, Fens and Yad were calculated by density functional theory (DFT) using the B3LYP function with the 6−31 G(d) basis set. As shown in Fig. 2, it was found that the LUMOs of the three emitters were mainly distributed in the acceptor moieties, and the HOMOs of Fene, Fens and Yad were mainly distributed in the donor moieties. These molecules showed highly twisted structures due to the larger steric hindrance. The HOMO/LUMO energy levels were calculated to be −5.03/−2.08 eV for Fene, −4.97/−1.86 eV for Fens and −5.19/−2.14 eV for Yad, respectively. The excited state features of Fene, Fens and Yad were further calculated based on the time-dependent DFT using the B3LYP function with the 6−31 G(d) basis set, respectively. The S1/T1 values were calculated to be −2.24/−2.23 eV for Fene, −2.50/−2.52 eV for Fens and −2.54/−2.56 eV for Yad, which gave the ΔEST values of 0.01, 0.02 and 0.02 eV for Fene, Fens and Yad, respectively. Obvious spatial separation between the HOMOs and LUMOs and the small ΔEST demonstrated that the reverse intersystem crossing (RISC) processes of T1 to S1 could be activated by the thermal energy of room-temperature.

|
Download:
|
| Fig. 2. The HOMO and LUMO of Fene, Fens and Yad obtained by the Gaussian 09 DFT B3LYP/6-31+G* level of theory. | |
The UV–vis spectra of Fene, Fens and Yad were measured in toluene with the concentration of 10−5 mol/L. As depicted in Fig. S7a (Supporting information), these emitters exhibited similar absorption bands due to their similar D-A-D structures and donor units. The absorption band in the range of 287–318 nm could be assigned to the locally excited (LE) transitions of the acridine, phenothiazine and phenoxazine moieties or the quinoline unit. Moreover, the lower energy absorptions ranging from 357 nm to 377 nm corresponded to the strong intramolecular charge-transfer (ICT) transitions from different donor units to quinoline moiety. Furthermore, the fluorescence spectra of Fene, Fens and Yad exhibited obvious solvatochromism effect. As shown in Fig. S8 (Supporting information), the solvatochromic effect resulted in the red-shifted emission with increased solvent polarity, indicating the emission features of ICT for the emitters. The emission bands of Fene, Fens and Yad centered at 584, 591 and 544 nm, and their absolute PLQYs in neat films were 58.24%, 36.11% and 79.63%, respectively.
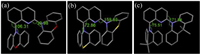
To obtain their experimental ΔEST values, we investigated the emission spectra of Fene, Fens and Yad in films at 77 K. Their fluorescence bands at 77 K were centered at 566, 563 and 543 nm, which resulted in S1 energy levels of 2.19, 2.20 and 2.28eV, respectively. Moreover, it was also found the phosphorescence bands of Fene, Fens and Yad were centered at 576, 570 and 552 nm (Table S4 in Supporting information), and their corresponding T1 energy levels were 2.15, 2.17 and 2.24 eV, respectively. Thus, the ΔEST values were estimated to be 0.04 eV for Fene, 0.03 eV for Fens and 0.04 eV for Yad. Small ΔEST could enable an efficient RISC process with a thermal aid for these compounds. In order to confirm the emission feature of Fene, Fens and Yad, transient PL spectra were also measured in neat films. As shown in Fig. 3, these emitters displayed the delayed fluorescence (DF) components with lifetimes of 2.75, 3.27 and 16.05 μs, respectively, which might be due to the thermal up-conversion of excitons from T1 to S1. Moreover, the temperature-dependent transient PL of Fene, Fens and Yad (Fig. 4) was then investigated. The ratio of the delayed component gradually increased from 77 K to 300 K, demonstrating the typical characteristics of TADF for these emitters. Excitation-emission fluorescence mappings of Fene, Fens and Yad were also carried out in neat films. It was further found that fluorescence intensity weakened with increasing excitation wavelength.
|
Download:
|
| Fig. 3. (a) Fluorescence spectra of Fene, Fens and Yad in neat films at room temperature; Transient PL spectra of (b) Fene, (c) Fens and (d) Yad in neat films at room temperature. | |

|
Download:
|
| Fig. 4. Steady-state excitation-emission mapping of (a) Fene, (b) Fens and (c) Yad in neat films at room temperature; the temperature-dependent transient photoluminescence spectra of (d) Fene, (e) Fens and (f) Yad in the neat films. | |
Thermogravimetric analyses (TGA) were also carried out to investigate the thermal stability of the emitters. As shown in Figs. S4–S6 (Supporting information), Fene, Fens and Yad exhibited good thermal stability with a 5% weight loss decomposition temperature (Td) of 360, 320 and 314 ℃, respectively. These results indicated that these luminogens possessed adequate thermal stability for the light-emitting devices.
Cyclic voltammetry (CV) was further used to investigate the electrochemical properties of the emitters. As shown in Fig. S7 (Supporting information), according to the onsets of oxidation and reduction potentials in their CV curves, the HOMO/LUMO levels of Fene, Fens and Yad were determined to be -5.90/-3.04 eV, -5.63/-2.53 eV and -6.09/-3.29 eV, respectively. These favorable HOMO/LUMO levels matched well with the widely used hole transporting material of 1, 1-bis[(di-4-tolylamino)phenyl]cyclohexane (TAPC) and electron-transporting layer of 1, 3, 5-tri(mpyrid-3-yl-phenyl)benzene (TmPyPB), which could facilitate the charge carrier injection and transportation from adjacent layer to the emissive layer.
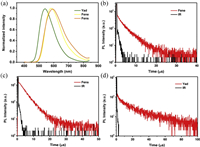
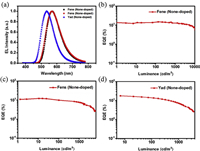
The PL spectra of emitters Fene, Fens and Yad in THF/water mixtures were measured to investigate their AIE properties. As shown in Fig. 5, the PL spectra of Fene, Fens and Yad showed negligible emission in dilute THF solution. However, when the value of water fraction reached to 80%, relatively strong fluorescence emissions were observed. With the continuing increase of water fraction, the fluorescence intensities enhanced gradually. When the value of water fraction reached to 90%, the fluorescence intensities of Fene, Fens and Yad were much stronger than those in THF, suggesting that the typical AIE characteristics existed. The transient PL curves of Fene, Fens and Yad were also measured in THF under air atmosphere at room temperature. Interestingly, when the water fraction varied from 80% to 90% in THF-water mixtures, the lifetimes and proportion of DF gradually increased with the increase of water fractions under air atmosphere at room temperature. Rotations of the aromatic rings and vibrations of Fene, Fens and Yad resulted in the conformational changes and thus influenced the RISC processes with the loss of DF in solution under air atmosphere. Long-lived luminescence under air atmosphere indicated that the triplet excited states in aggregate states were protected from oxygen, consequently leading to the effective RISC and delayed fluorescence emission. The outstanding emission characteristics of these materials could make them to have the great potential application as emitters in non-doped OLEDs. Thus, as shown in Fig. 6, the devices were fabricated with the structure of indium tin oxide (ITO)/(TAPC) (30 nm)/4, 4′, 4″-tri(N-carbazolyl)-triphenylamine (TCTA) (5 nm)/emission (15 nm)/(TmPyPB) (65 nm)/LiF (1 nm)/Al (100 nm), in which TAPC and TmPyPB were used as hole- and electron-transportation layers, respectively. Moreover, TCTA was inserted between TAPC and the emitting layer to reduce the hole-injection barrier and achieve step-by-step increments for each energy level. We further used LiF served as the electron-injection layer to eliminate the barrier caused by the interface electric double layer and reduce the Fermi level of the cathode. The non-doped OLEDs were fabricated by using co-deposited films of emission layers. As shown in Table 1, the non-doped optimal devices based on Fene, Fens and Yad displayed the maximum EL emissions at 570, 568 and 534 nm, respectively. Moreover, the non-doped devices of Fene, Fens and Yad showed excellent performances with the EQEmax of 14.9%, 13.1% and 17.4%, respectively. It was also found that the turn-on voltages of the non-doped devices are all low ranging from 3.0 V to 3.2 V.

|
Download:
|
| Fig. 5. PL spectra of (a) Fene, (b) Fens and (c) Yad in THF/water mixtures with different water fractions (fw); PL decay curves of (d) Fene, (e) Fens and (f) Yad in THF/water mixtures with different water fractions (fw) in air. | |
|
Download:
|
| Fig. 6. (a) Electroluminescence spectra of devices at 6 V; external quantum efficiency versus luminance curves for the devices of (b) Fene, (c) Fens and (d) Yad. | |
|
|
Table 1 Summary of EL data for the devices. |
In summary, we have conveniently synthesized three emitters Fene, Fens and Yad with quinoline as the new electron acceptor, which exhibited both TADF and AIE properties. Small ΔEST and twisted structures made them show good photoluminescence and AIDF properties, which could be greatly benefit for highly efficient non-doped OLEDs. Consequently, non-doped OLEDs based on the emitters were fabricated, which achieved excellent EQEmax of 14.9% and 13.1% with neat films of Fene and Fens, respectively. Moreover, the non-doped device based on the Yad neat film achieved the EQEmax of 17.4% owing to the good photoluminescence and obvious AIDF property. Notably, all devices exhibited relatively low turn-on voltages ranging from 3 V to 3.2 V. This represents the first highly efficient TADF emitters based on the quinoline electron acceptor. The results presented herein also suggested that the quinoline-based emitters could have a potential application in non-doped OLEDs for displays and solid-state lighting.
Declaration of competing interestWe declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentWe thank the National Natural Science Foundation of China (Nos. 91956119, 21871272, 21521002) for financial supports.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.07.041.
| [1] |
H. Uoyama, K. Goushi, K. Shizu, et al., Nature 492 (2012) 234-238. DOI:10.1038/nature11687 |
| [2] |
Q. Zhang, B. Li, S. Huang, et al., Nat. Photonics 8 (2014) 326-332. DOI:10.1038/nphoton.2014.12 |
| [3] |
Z. Yang, Z. Mao, Z. Xie, et al., Chem. Soc. Rev. 46 (2017) 915-1016. DOI:10.1039/C6CS00368K |
| [4] |
T. Huang, W. Jiang, L. Duan, J. Mater. Chem. C 6 (2018) 5577-5596. DOI:10.1039/C8TC01139G |
| [5] |
Y. Liu, C. Li, Z. Ren, et al., Nat. Rev. Mater. 4 (2018) 18020. |
| [6] |
J. Luo, S. Gong, Y. Gu, et al., J. Mater. Chem. C 4 (2016) 2442-2446. DOI:10.1039/C6TC00418K |
| [7] |
Y. Wang, Yu. Zhang, W. Hu, et al., ACS Appl.Mater. Interfaces 11 (2019) 26165-26173. DOI:10.1021/acsami.9b07005 |
| [8] |
P. Wei, D. Zhang, L. Duan, Adv. Funct. Mater. 29 (2019) 1907083. |
| [9] |
M. Li, Y. Liu, R. Duan, et al., Angew. Chem. Int. Ed. 56 (2017) 8818-8822. DOI:10.1002/anie.201704435 |
| [10] |
H. Liu, J. Zeng, J. Guo, et al., Angew. Chem. Int. Ed. 57 (2018) 9290-9294. DOI:10.1002/anie.201802060 |
| [11] |
D.H. Ahn, H. Lee, S.W. Kim, et al., ACS Appl. Mater. Interfaces 11 (2019) 14909-14916. DOI:10.1021/acsami.9b00931 |
| [12] |
F. Song, Z. Xu, Q. Zhang, et al., Adv. Funct. Mater. 28 (2018) 1800051. DOI:10.1002/adfm.201800051 |
| [13] |
M. Li, Y.F. Wang, D. Zhang, et al., Angew. Chem. Int. Ed. 59 (2020) 3500-3504. DOI:10.1002/anie.201914249 |
| [14] |
Y. Zhang, X. Zhang, H. Zhang, et al., J. Phys. Chem. C 123 (2019) 24746-24753. DOI:10.1021/acs.jpcc.9b07414 |
| [15] |
W. Li, B. Li, X. Cai, et al., Angew. Chem. Int. Ed. 58 (2019) 11301-11305. DOI:10.1002/anie.201904272 |
| [16] |
L.S. Cui, Y.L. Deng, D.P.K. Tsang, et al., Adv. Mater. 28 (2016) 7620-7625. DOI:10.1002/adma.201602127 |
| [17] |
D. Zhang, X. Song, M. Cai, et al., Adv. Mater. 30 (2018) 1705406. DOI:10.1002/adma.201705406 |
| [18] |
W. Chen, F. Song, Chin. Chem. Lett. 30 (2019) 1717-1730. DOI:10.1016/j.cclet.2019.08.032 |
| [19] |
M. Cai, D. Zhang, J. Xu, et al., ACS Appl. Mater. Interfaces 11 (2019) 1096-1108. DOI:10.1021/acsami.8b16784 |
| [20] |
Y.F. Wang, H.Y. Lu, C. Chen, et al., Org. Electron. 70 (2019) 71-77. DOI:10.1016/j.orgel.2019.03.020 |
| [21] |
D. Zhang, M. Cai, Y. Zhang, et al., Mater. Horiz. 3 (2016) 145-151. DOI:10.1039/C5MH00258C |
| [22] |
D.W. Zhang, M. Li, C.F. Chen, Chem. Soc. Rev. 49 (2020) 1331-1343. DOI:10.1039/C9CS00680J |
| [23] |
Z.G. Wu, H.B. Han, Z.P. Yan, et al., Adv. Mater. 31 (2019) 1900524. DOI:10.1002/adma.201900524 |
| [24] |
X. Chen, J.W. Zhao, X.H. Zheng, et al., Chin. Chem. Lett. 30 (2019) 1989-1993. DOI:10.1016/j.cclet.2019.09.013 |
| [25] |
W. Yuan, H. Yang, M. Zhang, et al., Chin. Chem. Lett. 30 (2019) 1955-1958. DOI:10.1016/j.cclet.2019.08.019 |
| [26] |
C. Li, R.S. Nobuyasu, Y. Wang, et al., Adv. Opt. Mater. 5 (2017) 1700435. DOI:10.1002/adom.201700435 |
| [27] |
M. Zhang, L. Chen, X. Xu, et al., J. Mater. Chem. C 7 (2019) 9850-9855. DOI:10.1039/C9TC03013A |
| [28] |
J. Guo, Z. Zhao, B.Z. Tang, Adv. Opt. Mater. 6 (2018) 1800264. DOI:10.1002/adom.201800264 |
| [29] |
J. Huang, H. Nie, J. Zeng, et al., Angew. Chem. Int. Ed. 56 (2017) 12971-12973. DOI:10.1002/anie.201706752 |
| [30] |
Z. Huang, Z. Bin, R. Su, et al., Angew. Chem. Int. Ed. 59 (2020) 9992-9996. DOI:10.1002/anie.201915397 |
| [31] |
J. Guo, X.L. Li, H. Nie, et al., Chem. Mater. 29 (2017) 3623-3631. DOI:10.1021/acs.chemmater.7b00450 |
| [32] |
J. Guo, Z. Zhao, B.Z. Tang, Adv. Opt. Mater. 6 (2018) 1800264. DOI:10.1002/adom.201800264 |
| [33] |
R. Komatsu, H. Sasabe, Y. Seino, et al., J. Mater. Chem. C 4 (2016) 2274-2278. DOI:10.1039/C5TC04057D |
| [34] |
J. Li, T. Nakagawa, J. MacDonald, et al., Adv. Mater. 25 (2013) 3319-3323. DOI:10.1002/adma.201300575 |
| [35] |
K. Shizu, H. Tanaka, M. Uejima, et al., J. Phys. Chem. C 119 (2015) 1291-1297. DOI:10.1021/jp511061t |
| [36] |
K. Wu, T. Zhang, L. Zhan, et al., Adv. Opt. Mater. 4 (2016) 1558-1566. DOI:10.1002/adom.201600304 |
| [37] |
A. Endo, K. Sato, K. Yoshimura, et al., Appl. Phys. Lett. 98 (2011) 083302. DOI:10.1063/1.3558906 |
| [38] |
Y.F. Wang, H.Y. Lu, Y.F. Shen, et al., Chem. Commun. 55 (2019) 9559-9562. DOI:10.1039/C9CC04995A |
| [39] |
K. Kawasumi, T. Wu, T. Zhu, et al., J. Am. Chem. Soc. 137 (2015) 11908-11911. DOI:10.1021/jacs.5b07932 |
| [40] |
S. Wang, X. Yan, Z. Cheng, et al., Angew. Chem. Int. Ed. 54 (2015) 13068-13072. DOI:10.1002/anie.201506687 |
| [41] |
P. Data, P. Pander, M. Okazaki, et al., Angew. Chem. Int. Ed. 55 (2016) 5739. DOI:10.1002/anie.201600113 |
| [42] |
C. Chen, H.Y. Lu, Y.F. Wang, et al., J. Mater. Chem. C 7 (2019) 4673-4680. DOI:10.1039/C9TC00503J |
| [43] |
F. Cottet, M. Marull, O. Lefebvre, et al., Eur. J. Org. Chem. 8 (2003) 1559-1568. |
 2021, Vol. 32
2021, Vol. 32