b Key Laboratory of Special Functional Aggregated Materials, Ministry of Education, Shandong University, Ji'nan 250100, China
Organic fluorescent materials have attracted much attention because of their potential applications in fluorescent sensors [1-3], light-emitting diodes [4-6], smart color-changing materials, fluorescent probes [7-9] and imaging agents in biological systems [10, 11]. Organic fluorescent small molecules are an important way to obtain new luminescent materials. Due to the hydrophobic effect of organic chromophores in aqueous solution, most of the organic fluorescent small molecules are conjugated molecules with poor water solubility. However, the biological system is a relatively complex aqueous system. Therefore, it greatly hinders the application of organic fluorescent small molecules in fluorescence imaging [12]. Moreover, organic fluorescent molecules with aromatic rings have toxicity [13]. Therefore, it is a great challenge to obtain water-soluble organic fluorescent molecules with good biocompatibility and apply them in biomedical field.
Supramolecular assembly can change the optical properties of fluorescent molecules in aqueous solution by simply changing the noncovalent interactions such as coordinative bonds [14-16], hydrogen bonding [17, 18], electrostatic interactions [19-21], and host-guest interactions [22-24]. Cucurbit[7]uril (CB[7]) is a kind of macrocyclic host molecule containing seven glycoluril units bridged by methylene group, with a cavity diameter of 7.3Å and a portal diameter of 5.4Å [25-29]. CB[7] can not only form inclusion complexes with some fluorescent molecules with high selectivity and high binding constant in aqueous solution, but also affect the optical properties of organic guest molecules through encapsulation [30-34]. Galoppini and coworkers investigated the effect of CB[7] encapsulation on the fluorescence and electrochemical properties of p-tolyl viologen, DTV2+ [32]. They found that the fluorescence emission intensity of the DTV2+@2CB[7] complexes was an order of magnitude higher than free DTV2+. Trabolsi and coworkers employed pseudorotaxanes that formed by CB[7] and a diamino-viologen linker condensed with an aromatic tri-aldehyde core to prepare polyrotaxanated covalent organic framework (COF) [35]. Under the excitation of 375 nm, the COF, TpVCB[7], showed bright blue luminescence with λmax = 467 nm, while the COF (TpV) without CB[7] showed very weak luminescence. With CB[7] encapsulation, the fluorescence emission intensity of TpVCB[7] is eight times higher than that of TpV. These enhancement are mainly due to the fact that CB[7] encapsulation limits the molecular torsion and increases the molecular rigidity.
Fluorescent molecules need good biocompatibility if they are to be used for biological applications requiring fluorescence, such as in fluorescent probes and sensors and in bio-imaging. However, there are few reports in the literature on the effect of host-guest interaction on the biocompatibility of fluorescent guest molecules. Scherman and coworkers reported the formation of the pyrene-viologen-CB[8] ternary complex. They controlled the release and encapsulation of viologen by controlling the association and dissociation of the supramolecular peptide amphiphile, to modulate the toxicity and non-toxicity of supramolecular system [36]. Zhang and his colleagues proposed a supramolecular chemotherapy. They employed dynamic CB[7]-mediated host-guest interaction to control the loading and releasing of antitumor drugs, so as to regulate the cytotoxicity of antitumor drugs and improve the efficiency of antitumor drugs [37, 38]. Inspired by these work, we proposed that CB[7] can not only change the fluorescence performance of guest molecules, but also change the biocompatibility of fluorescent guest molecules, making it a wide application prospect in the field of biosensor.
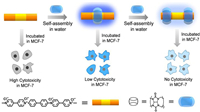
In order to prove the above hypothesis, we synthesized the bis-viologen biphenyl molecule (BPV22+) [39] and studied the effect of CB[7] on the fluorescence performance and biocompatibility of BPV22+. Through CB[7] encapsulation, not only the fluorescence intensity of BPV22+ increased significantly, but also the cytotoxicity of BPV22+ decreased markedly (Scheme 1). After interaction with CB[7], BPV22+ has no cytotoxicity at all. Our main objective is to reveal how host-guest interaction affects the fluorescent properties and the cytotoxicity of BPV22+, expand the biological application value of fluorescent molecules, and make more molecules used for biological imaging and labeling. Our group has designed fluorescent molecular switch based on the host-guest interaction [40]. we can combine the above ideas to design an integrated component of diagnosis and treatment in the future.
|
Download:
|
| Scheme 1. Schematic illustration of the assembly modes of BPV22+ and CB[7]. | |
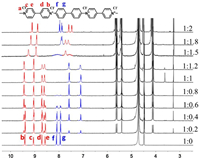
The formation of the host-guest complexes was monitored by 1H NMR titration in D2O (Fig. 1 and Fig. S2 in Supporting information). The signals of protons sitting inside the CB[7] cavity undergo significant upfield shifts, and the signals of protons close to the cavity portal, but not included, undergo a slight downfield shift. These characteristics provide important insight into the detection of the binding site of BPV22+ with CB[7].
|
Download:
|
| Fig. 1. 1H NMR spectra of BPV22+ with CB[7] in D2O. | |
Upon addition of 1.0 equiv. of CB[7], the signals corresponded to the protons of the biphenyl moiety (Hf and Hg) shifted upfield by 0.52 and 0.86 ppm, whereas the signals of the viologen moiety protons (Hb, Hc, Hd and He) slightly shifted downfield by 0.02 ppm. The singlet associated with the methyl group (Ha) of BPV22+ remained unchanged upon the addition of CB[7]. These results indicated that BPV22+ could form a binary complex with CB[7], which resided on the biphenyl moiety. Upon addition of 2.0 equiv. of CB[7], the proton signals continued to change significantly except for the viologen moiety protons (Ha). The signals corresponded to the viologen moiety protons (Hd and He) shifted upfield by 1.10 ppm, whereas the signals of the viologen moiety protons (Hb and Hc) and biphenyl moiety (Hf and Hg) slightly shifted upfield by 0.31, 0.17, 0.12 and 0.08 ppm, respectively.
We can clearly see a remarkable broadening of the proton signals before the amount of CB[7] reached 2.0 equiv. (Fig. 1). A similar broadening of the 1H NMR spectra for CB[7] complexes of viologens has been reported previously by Kaifer and coworkers [41, 42]. A reasonable explanation for this phenomenon is that there is a dynamic change among species during the time scale of NMR observation [43]. This equilibria include shuttling the host between the viologen moiety and biphenyl moiety. The1H NMR peak of the BPV22+@2CB[7] was sharper, and the extra amounts of CB[7] did not caused significant spectral changed, demonstrating the complexation was saturated with 2.0 equiv. of CB[7]. In summary, the 1H NMR data indicated that different complexes formed when the ratio of BPV22+ and CB[7] was different. The binary complex BPV22+@CB[7] was formed upon addition of 1.0 equivalent of CB[7], however, a ternary complex BPV22+@2CB[7] was formed upon addition of 2.0 equiv. of CB[7].
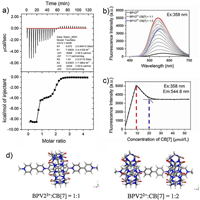
The binding properties of BPV22+@CB[7] was studied using isothermal titration calorimetry (ITC). A titration curve was shown in Fig. 2a. The titration plot was fitted with two sets of sites model, with first-step binding constant of (7.26±4.41) × 108 L/mol and second-step binding constant of (5.63±0.74) × 105 L/mol.
|
Download:
|
| Fig. 2. (a) ITC titration plot of 2.0 mmol/L CB[7] with 0.1 mmol/L BPV22+ at 298 K in H2O. (b) Emission spectra of the titration of BPV22+ (10 μmol/L) with CB[7] (0-200 μmol/L). (c) The fluorescence intensity at 544.8 nm with different amount of CB[7]. (d) Simulation model of the 1:1 and 2:1 complex of CB[7] and BPV22+. | |
To futher explore the interaction between BPV22+ and CB[7], the fluorescence titration curves of BPV22+ (10 μmol/L) with various CB[7] concentrations in aqueous solution were obtained. As shown in Fig. 2b, aqueous solution of BPV22+ in the absence of CB[7] showed weak fluorescence. When an equivalent CB[7] was added, however, the emission spectra showed that the fluorescence emission intensity increased significantly, and the emission band blue shifted of 28 nm (λem from 571 nm to 543 nm). When two equivalent CB[7] were added, the fluorescence emission intensity decreased slightly, accompanied by the red shift of emission peak (λem from 542.8–553.8 nm). The titration curve of Fig. 2c showed that the fluorescence emission intensity reached maximum when the ratio of host to guest was 1:1, and the fluorescence emission intensity no longer changed when the ratio of host and guest reached 2:1. The results showed that the CB[7] encapsulation resulted in an order of magnitude enhancement of the fluorescence emission intensity.
This fluorescence intensity enhancement and blue shift of the emission induced by encapsulation of the guest molecule in a host has been reported in the literature [32]. This is mainly due to the following reasons. 1) The encapsulation of CB[7] increases the rigidity of the molecule and leads to a fluorescence enhancement. On the one hand, with the increase of rigidity, the intramolecular vibration is weakened, which makes the excitation energy of the molecule difficult to release in the form of thermal energy due to vibration. On the other hand, the increase of intramolecular vibration is weakened, which makes the excitation energy of the molecule difficult to release in the form of thermal energy due to vibration. On the other hand, the increase of rigidity is conducive to improving the coplanarity of molecules and fluidity of intramolecular π electrons. 2) The encapsulation of CB[7] can protect BPV22+ from being quenched by solvent molecules. To better prove the above conclusion, the main dihedral distributions of BPV22+ molecule were investigated in both free state and the complex state with CB[7]. The results of dihedral distrabutions were shown in Fig. 2d and Fig. S8 (Supporting information).
As shown in Table S1 (Supporting information), the fluorescence quantum yield of BPV22+ was measured in water in the presence of different equivalents of CB[7]. The aqueous solution of BPV22+ exhibited a low fluorescence quantum yield (Φ=3.25%) in the absence of CB[7]. The fluorescence quantum yield and lifetime were significantly increased upon the addition of CB[7], depending on the ratio of BPV22+ and CB[7]. When 1.0 equiv. of CB[7] was added, the maximum fluorescence quantum yield (Φ=19.45%) was obtained, when 2.0 equiv. of CB[7] were added, the fluorescence quantum yield decreased slightly (Φ=15.11%). The time-resolved experiments showed that the excited-state lifetime of BPV22+ increased significantly from 0.05ns to 1.04ns upon encapsulation with 1.0 equiv. of CB[7] (Fig. S5 and Table S1). When CB[7] were added to 2.0 equiv., the excited-state decay kinetics of BPV22+ displayed a long lifetime of 1.2ns with a relatively short lifetime of 0.6ns. The fluorescence decay profiles obey a biexponential function, proving the presence of two species in solution. For BPV22+, the longer lifetime component of ~1.0ns in aqueous solution was associated with the BPV22+@CB[7], the shorter lifetime component of ~0.5ns in aqueous solution was associated with the BPV22+@2CB[7]. Due to the reversible assembly of BPV22+@CB[7] and BPV22+@2CB[7], there was a dynamic change among species. The contribution rate of BPV22+@CB[7] was 45.7%, the contribution rate of BPV22+@2CB[7] was 54.3%.
Upon addition of 1.0 equiv. of CB[7], the radiative decays rate had a mild change, from 19.95 s−1 to 0.18 s−1, and the non-radiative decays rate was much slower, from 19.95 s−1 to 0.76 s−1, calculated from the fluorescence quantum yield and lifetime. The latter were caused by a lack of fluorophore aggregation after encapsulation by CB[7], and an inhibition of nonradiative relaxation by interaction with the solvent. Overall, the results indicated that the confinement in CB[7] primarily affects the rate of non-radiative energy dissipation processes of the singlet-excited BPV22+.
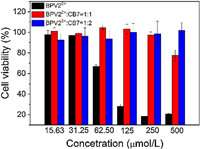
To explore the effect of CB[7] encapsulation on the biocompatibility of BPV22+, we used the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay to studied the in vitro toxicity of BPV22+. MTT assays were performed after MCF-7 cells were incubated with different concentrations of BPV22+ and BPV22+@CB[7] and BPV22+@2CB[7] for 48h. Fig. 3 showed that when BPV22+ was encapsulated in the CB[7] cavity, the cytotoxicity of BPV22+ is low. After a 48h incubation with 125 μmol/L BPV22+, the viability of the cells was reduced to 28%, whereas for the BPV22+@CB[7] and BPV22+@2CB[7], the viability remained at 100%. At a 500 μmol/L loading concentration of BPV22+, the cell viability reduced to 20%, the viability of BPV22+@CB[7] was also reduced to 77%, and the activity of BPV22+@2CB[7] was still maintained at 100%.
|
Download:
|
| Fig. 3. Effect of different concentrations of BPV22+, BPV22+@CB[7], and BPV22+@2CB[7] on MCF-7 cells viability after 48 h incubation. The cell viability was assessed using the MTT assay. The values presented are the mean ± SD (n = 3). | |
Some small molecules containing amines, amides, aromatic rings and pyridines tend to bind double-stranded DNA in different ways, including electrostatic adsorption and intercalation between base pairs. These interactions hinder the function of various enzymes and thereby induce toxicity to the living cells [44]. BPV22+ is a toxic substance for humans and animals. We think that when BPV22+ was encapsulated by 1.0 equiv. of CB[7], the host molecule prevented the insertion of BPV22+ between the base pairs, but the viologen moiety still had electrostatic adsorption, so it still induced toxicity to living cells. When BPV22+ was encapsulated by 2.0 equiv. of CB[7], its interaction with DNA was completely prevented, which had no effect on cell viability.
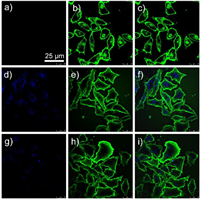
Based on the MTT results, 62.5 μmol/L BPV22+ with various concentration of CB[7] were used for imaging experiments. To understand the ability of BPV22+ to be internalized by cells, MCF-7 cells were incubated with BPV22+, BPV22+@CB[7] and BPV22+@2CB[7] for 6h, respectively, following by imaged with a confocal laser scanning microscopy (CLSM, Leica SP8). As Fig. 4 showed, there was a significant difference between BPV22+ and BPV22+@CB[7]. The image of BPV22+ did not show obvious color, while the images of BPV22+@CB[7] obtained show a bright blue emission in the cytoplasm of the cells without changing their morphologies. The image of BPV22+@2CB[7] showed a relatively low-bright blue emission. The phenomenon was consistent with the change in the fluorescence emission intensity.
|
Download:
|
| Fig. 4. Live-cell confocal microscopy images of MCF-7 cells. Cells were incubated with (a) BPV22+, (d) BPV22+@CB[7] and (g) BPV22+@2CB[7] (62.5 μmol/L in PBS solution). (b, e, h) Cells were incubated with WGA. Cells were incubated with WGA and (c) BPV22+ (f) BPV22+@CB[7] and (i) BPV22+@2CB[7]. Scale bars: 25 μm. | |
To further confirm the intracellular internalization and location of BPV22+, cells incubated with BPV22+ for 6h were further treated by WGA, staining cell membrane. The CLSM image revealed that the BPV22+ mainly located inside the cytoplasm other than the nuclei and membranes (Fig. 4), confirming the efficient internalization and release behavior of BPV22+.
In summary, a bi-viologen rigid guest molecule BPV22+ can form 1:1 or 1:2 inclusion compounds with CB[7] in aqueous solution. Upon encapsulated by 1.0 equiv. CB[7], the emission intensity of the BPV22+@CB[7] complex was enhanced 1 order of magnitude (Φ=19.45%, τ = 1.04ns) and blue-shifted by 28 nm. Upon encapsulated by 2.0 equivalent CB[7], the emission intensity of the BPV22+@2CB[7] complex decreased (Φ=15.11%, τ1=1.2 ns, τ2=0.6 ns). Meanwhile, the cytotoxicity of BPV22+ was significantly reduced by the encapsulation of CB[7]. This remarkable enhancement of fluorescence intensity and biocompatibility makes BPV22+ performed well in cell imaging, which holds promise for the application of fluorescent molecules in biological fields such as biological imaging and labeling.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21374055) and the Fundamental Research Funds of Shandong University (No. 11190078614092).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2020.07.039.
| [1] |
K.P. Carter, A.M. Young, A.E. Palmer, Chem. Rev. 114 (2014) 4564-4601. DOI:10.1021/cr400546e |
| [2] |
D. Cao, H. Meier, Chin. Chem. Lett. 30 (2019) 1758-1766. DOI:10.1016/j.cclet.2019.06.026 |
| [3] |
N. Yan, J. Song, F. Wang, Chin. Chem. Lett. 30 (2019) 1984-1988. DOI:10.1016/j.cclet.2019.09.039 |
| [4] |
M.Y. Wong, E. Zysman-Colman, Adv. Mater. 29 (2017) 1605444. DOI:10.1002/adma.201605444 |
| [5] |
M. Zhu, C. Yang, Chem. Soc. Rev. 42 (2013) 4963-4976. DOI:10.1039/c3cs35440g |
| [6] |
J.H. Jou, S. Kumar, A. Agrawal, J. Mater. Chem. C 3 (2015) 2974-3002. DOI:10.1039/C4TC02495H |
| [7] |
Y.L. Pak, K.M.K. Swamy, J. Yoon, Sensors 15 (2015) 24374-24396. DOI:10.3390/s150924374 |
| [8] |
J.S. Kim, D.T. Quang, Chem. Rev. 107 (2007) 3780-3799. DOI:10.1021/cr068046j |
| [9] |
Y. Luo, W. Zhang, M. Liu, et al., Chin. Chem. Lett. 32 (2021) 367-370. DOI:10.1016/j.cclet.2020.02.023 |
| [10] |
S.K. Yang, X. Shi, S. Park, et al., Nat. Chem. 5 (2013) 692-697. DOI:10.1038/nchem.1706 |
| [11] |
H. Lu, J. Mack, Y. Yang, et al., Chem. Soc. Rev. 43 (2014) 4563-4601. DOI:10.1039/C4CS00051J |
| [12] |
M. De, P.S. Ghosh, V.M. Rotello, Adv. Mater. 20 (2008) 4225-4241. DOI:10.1002/adma.200703183 |
| [13] |
X. Michalet, F.F. Pinaud, L.A. Bentolila, et al., Science 307 (2005) 538-544. DOI:10.1126/science.1104274 |
| [14] |
Q. Tang, S. Liu, Y. Liu, et al., Inorg. Chem. 53 (2013) 289-293. |
| [15] |
M.S. Wang, G.C. Guo, Chem. Commun. 52 (2016) 13194-13204. DOI:10.1039/C6CC03184F |
| [16] |
X. Yan, T.R. Cook, P. Wang, et al., Nat. Chem. 7 (2015) 342. DOI:10.1038/nchem.2201 |
| [17] |
H. Wang, X. Ji, Z. Li, et al., Mater. Chem. Frontiers 1 (2017) 167-171. DOI:10.1039/C6QM00164E |
| [18] |
D. Gonza'lez-Rodri'guez, A.P. Schenning, Chem. Mat. 23 (2010) 310-325. |
| [19] |
K. Sakai, S. Tsuchiya, T. Kikuchi, et al., J. Mater. Chem. C. 4 (2016) 2011-2016. DOI:10.1039/C5TC04290A |
| [20] |
X.H. Jin, C. Chen, C.X. Ren, et al., Chem. Commun. 50 (2014) 15878-15881. DOI:10.1039/C4CC07063A |
| [21] |
S. Bhattacharya, S.K. Samanta, Chem. Eur. J. 18 (2012) 16632-16641. DOI:10.1002/chem.201201940 |
| [22] |
S.H. Li, X. Xu, Y. Zhou, et al., Org. Lett. 19 (2017) 6650-6653. DOI:10.1021/acs.orglett.7b03377 |
| [23] |
Y. Xia, S. Chen, X.L. Ni, ACS Appl. Mater. Interfaces. 10 (2018) 13048-13052. DOI:10.1021/acsami.8b02573 |
| [24] |
E. Masson, M. Raeisi, K. Kotturi, Isr. J. Chem. 58 (2018) 413-434. DOI:10.1002/ijch.201700120 |
| [25] |
J. Kim, I.S. Jung, S.Y. Kim, et al., J. Am. Chem. Soc. 122 (2000) 540-541. DOI:10.1021/ja993376p |
| [26] |
J.W. Lee, S. Samal, N. Selvapalam, et al., Acc. Chem. Res. 36 (2003) 621-630. DOI:10.1021/ar020254k |
| [27] |
J. Lagona, P. Mukhopadhyay, S. Chakrabarti, L. Isaacs, Angew. Chem. Int. Ed. 44 (2005) 4844-4870. DOI:10.1002/anie.200460675 |
| [28] |
A. Day, A.P. Arnold, R.J. Blanch, et al., J. Org. Chem. 66 (2001) 8094-8100. DOI:10.1021/jo015897c |
| [29] |
D. Shetty, J.K. Khedkar, K.M. Park, et al., Chem. Soc. Rev. 44 (2015) 8747-8761. DOI:10.1039/C5CS00631G |
| [30] |
H.J. Kim, W.S. Jeon, Y.H. Ko, K. Kim, PNAS 99 (2002) 5007-5011. DOI:10.1073/pnas.062656699 |
| [31] |
M. Freitag, E. Galoppini, Langmuir 26 (2010) 8262-8269. DOI:10.1021/la904671w |
| [32] |
M. Freitag, L. Gundlach, P. Piotrowiak, E. Galoppini, J. Am. Chem. Soc. 134 (2012) 3358-3366. DOI:10.1021/ja206833z |
| [33] |
Z.L. Qian, X.H. Huang, Q.C. Wang, Dye. Pigm. 145 (2017) 365-370. DOI:10.1016/j.dyepig.2017.02.042 |
| [34] |
W. Wu, S. Song, X. Cui, et al., Chin. Chem. Lett. 29 (2018) 95-98. DOI:10.1016/j.cclet.2017.08.049 |
| [35] |
G. Das, S.K. Sharma, T. Prakasam, et al., Commun. Chem. 2 (2019) 106. DOI:10.1038/s42004-019-0207-3 |
| [36] |
D.Z. Jiao, J. Geng, X.J. Loh, et al., Angew. Chem. Int. Ed. 51 (2012) 9633-9637. DOI:10.1002/anie.201202947 |
| [37] |
Y. Chen, Z. Huang, H. Zhao, et al., ACS Appl. Mater. Interfaces. 9 (2017) 8602-8608. DOI:10.1021/acsami.7b01157 |
| [36] |
D.Z. Jiao, J. Geng, X.J. Loh, et al., Angew. Chem. Int. Ed. 51 (2012) 9633-9637. DOI:10.1002/anie.201202947 |
| [37] |
Y. Chen, Z. Huang, H. Zhao, et al., ACS Appl. Mater. Interfaces. 9 (2017) 8602-8608. DOI:10.1021/acsami.7b01157 |
| [38] |
Y. Chen, Z. Huang, J.F. Xu, et al., ACS Appl. Mater. Interfaces. 8 (2016) 22780-22784. DOI:10.1021/acsami.6b08295 |
| [39] |
T.G. Zhan, T.Y. Zhou, F. Lin, et al., Org. Chem. Front. 3 (2016) 1635-1645. DOI:10.1039/C6QO00298F |
| [40] |
H. Chen, H. Yang, W.C. Xu, et al., Chin. Chem. Lett. 24 (2013) 857-860. DOI:10.1016/j.cclet.2013.05.028 |
| [41] |
K. Moon, A.E. Kaifer, et al., Org. Lett. 6 (2004) 185-188. DOI:10.1021/ol035967x |
| [42] |
R. Wang, D. Bardelang, M. Waite, et al., Org. Biomol. Chem. 7 (2009) 2435-2439. DOI:10.1039/b903057c |
| [43] |
P. Montes-Navajas, A. Corma, H. Garcia, J. Mol. Catalysis A: Chem. 279 (2008) 165-169. DOI:10.1016/j.molcata.2007.08.007 |
| [44] |
I. Roy, S. Bobbala, J. Zhou, et al., J. Am. Chem. Soc. 140 (2018) 7206-7212. DOI:10.1021/jacs.8b03066 |
 2021, Vol. 32
2021, Vol. 32 

