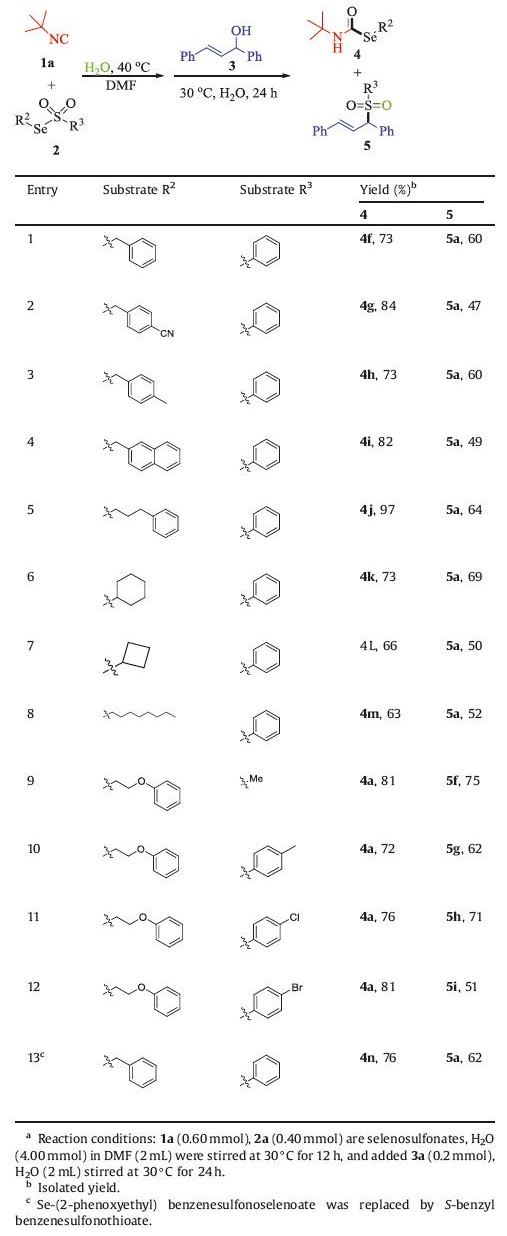
Sulfur and selenium play a prominent role in organic synthesis [1], and widely exist in natural products, bioactive molecules and organic materials [2]. In 2005, Takahashi's group reported the selenocarbamates could be used as effective superoxide anion radical scavengers in vitro [3]. Govindasamy and his colleagues synthesized spirodiazaselenurane by spirocyclization of corresponding aryl selenides for the first time, and found that the anti-inflammatory compound ebselen has antioxidant activity [4]. Masahiro and co-workers found benzo[b]benzo[4,5]thieno[2,3-d]thiophene (BTBT) is the basic structural unit of organic semiconductor materials [5]. Therefore, the construction of C−S and C–Se bonds is of strategic importance in current synthetic chemistry. In most of the explorations, selenium powder and diselenide were employed as starting material to construct the C–Se bond [6], and thioalcohol, inorganic sulfur and disulphide, to construct the C–S bond, respectively [7]. For example, Sonda's group reported a multicomponent reaction of alkyl halides, amide, carbon monoxide and selenium powder to construct selenocarbamates [8]. However, the selenium powder used in the reaction is sensitive to air and moisture, and has certain toxicity. Buchwald's team used thioalcohol to react with aryl halogenated hydrocarbons to construct aryl thioether compounds, but the thioalcohol used was irritating and toxic [9]. In recent years, as a new type of selenium and sulfur reagent, thiosulfonate and selenosulfonate are more stable and less toxic than the traditional selenium and sulfur reagents, Promoting them to be applied in a variety of organic transformations as pivotal synthetic building blocks. Traditionally, sulfonyl groups are often removed in the form of sulfinic acid or sodium sulfite, when thiosulfonates and selenosulfonates react with nucleophilic reagents (Scheme 1a) [10]. In 2016, Xu and his co-workers reported the construction of o‑alkynyl arylsulfides from aryne, alkyne, and benzenesulfonothioate [11]. Unfortunately, the sulfonyl group was deserted as a by-product. Our group reported the synthesis of selenocarbamates through metal-free multicomponent reactions of isocyanides, selenosulfonates, in the present of water [12]. Unavoidably, in this reaction, sulfonyl group merely left in the form of sulfinic acid.

|
Download:
|
| Scheme 1. One-pot two-step reaction. | |
Sulfones exist widely in many bioactive natural products as well as some of the best-selling drugs [13]. The most common drugs are Eletriptan ([2H]-SB-3CT) for the treatment of migraine headache and MMP-2, MMP-9 inhibitor for the treatment of prostate cancer [14]. On one hand, sulfuryl exhibits good nucleophilicity. Consequently, in some reactions involving thiosulfonates and selenosulfonates, the separated sulfonyl groups can react with other electrophilic reagents in the system (Scheme 1b) [15]. For instance, In 2005, Shi's group reported the DABCO-catalyzed addition of selenosulfonates to α, β-unsaturated ketones to generate α-selenylated ketones [16]. In this case, the sulfonyl group leaves at first, but eventually participates in the β-difunctionalization process. Similarly, in 2018, Xu and his co-workers realized oxidative trifunctionalization of olefins by thiosulfonates with the copper catalyst, ensuring the full utilization of thiosulfonates [17]. On the other hand, sulfinic acid and sodium sulfite can also be used in the synthesis of sulfonyl compounds directly. In 2005, Lu's group synthesized β-fluorosulfonyl compounds by the reaction of styrene with aryl sulfite under the catalysis of palladium [18]. Three years later, Loh et al. successfully constructed the allyl sulfone compounds, through the water-catalyzed reaction of sulfonic acid and allyl alcohol [19].
Over the years, practices of synthesis have generally relied on assembling several synthesis operation processes in a stop-and-go process. But we should notice the stop-and-go practice not only compromises the overall efficiency of the synthesis process, but also results significant amounts of waste, particularly organic solvents. As a continuation of our interests in green chemistry, we focus on developing effective ways to transform sulfinic acid, a by-product in the reaction of traditional selenosulfonates or thiosulfonates, into other useful substances without stop-and-go process. Therefore, we design a one-pot two-step reaction of selenosulfonates, isocyanides and allyl alcohols under aqueous conditions to afford selenocarbamates and allyl sulfone compounds. Through this novel design, we not only achieved the goal of killing two birds with one stone, but also greatly improved the atomic economy of the reaction.
The study was commenced by investigating the reaction of commercially available 2-isocyano-2-methylpropane 1a, Se-(2-phenoxyethyl)benzenesulfonoselenoate 2a with 1,3-diphenyl-2-enol 3a. The reaction was carried out in one-pot two-step way. The system after the reaction of 2-isocyano-2-methylpropane (1a) with Se-(2-phenoxyethyl)benzenesulfonoselenoate (2a) in the presence of water, and added 1,3-diphenyl-2-enol (3a) and 2mL water directly to it without separated. To our delight, we could obtain 4a and 5a in 87% and 44% yield, respectively. Then, we hope to increase the yield of the product 5a mainly by changing the conditions. Firstly, we investigated a series of solvents added in the second step (Table 1, entries 1–6). It was found that the desired products 4a could be obtained in moderate to good yields, but the yield of 5a has no obvious change, except that the addition of 0.5mL water resulted in lower yields of 4a and 5a products. Subsequently, we gradually increased the amount of 3a to observe the reaction efficiency (Table 1, entries 7–9). It shows us that when 3a was 0.4mmol, the reaction effect was the best, and the desired products 4a and 5a could be obtained in 90% and 65% yield, respectively. Then, we extended the reaction time of the second step to 48h, but the reaction efficiency did not change significantly (Table 1, entry 10). Finally, we adjusted the reaction temperature of the second step (Table 1, entries 11–13). We found that increasing the temperature would decompose the product 4a, and the yield of 5a was not improved. However, reducing the temperature could not consume the selenosulfonate completely and reduce the reaction effect. Therefore, 30 ℃ was the most suitable reaction temperature.
|
|
Table 1 Optimization of the reaction conditions.a |
With the optimized conditions in hand, we explored the substrate scope of various isocyanides 1 (Table 2). To our delight, we could obtain 4a and 5a in 90% and 65% yields. Alkyl isocyanides, including (1R, 3S, 5R, 7R)-2-isocyanoadamantane, could react smoothly under standard conditions. The desired products (4b-4e) and 5a were obtained in moderate yields. When aromatic isocyanides were employed in the reactions, we could obtain a small amount of the desired product.
|
|
Table 2 Substrate scope of isocyanides and allylic alcohols.a |
Subsequently, the scope of the various simple allylic alcohols 3 was explored (Table 2). A fluorine at the ortho- and para-position of 2-phenyl respectively provided the desired products 4a in 84% and 88% yields, while product 5b and 5d in relatively low yields of 50% and 41%. By comparison, when the substituent group is methyl, we could obtain the desired products in medium to excellent yields (4a and 5c, 5e).
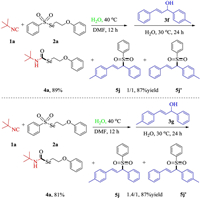
When we used (E)-1-phenyl-3-(p-tolyl)prop-2-en-1-ol (3f) as the substrate, We can obtain the target product 4a with a separation yield of 89% and a mixture of target products 5j and 5j' was obtained with a separation yield of 87%. According to the nuclear magnetic hydrogen spectrum, we analyzed that the ratio of 5j and 5j' in the mixture is about 1/1. Replacing 3f with 3g can also obtain the target product 4a in 81% yield, and a mixture of 5j and 5j' in 87% separation yield. But the ratio of the mixture has changed a little, and it has become 1.4/1 (Scheme 2).
|
Download:
|
| Scheme 2. Scope of allylic alcohols. Reaction conditions: 1a (0.60 mmol), 2a (0.40 mmol), H2O (4.00 mmol) in DMF (2 mL) were stirred at 30 ℃ for 12 h, and added 3f or 3g (0.2 mmol), H2O (2 mL) stirred at 30 ℃ for 24 h. | |
We further studied the substrate scope using a series of selenosulfonates 2 with different R2 and R3 groups (Table 3). Different substituted Se-benzyl benzenesulfonoselenoates were employed in the reactions. The desired products could be isolated in 47%–84% yields (4f-4i and 5a). To our delight, Se-(3-phenylpropyl)benzenesulfonoselenoate was proven to be suitable candidates for the reaction. The desired products 4j and 5a were isolated in 97% and 64% yields, respectively. Branched and cyclane-substituted selenosulfonates were compatible with the mild reaction conditions as well, leading to the desired products in moderate yields. When we replace selenosulfonate with S-benzyl benzenesulfonothioate, the reaction could proceed smoothly, and could obtain the desired product 5a and 4n in the yields of 76% and 62%.
|
|
Table 3 Substrate scope of allylic alcohols.a |
In summary, we have developed a one-pot two-step reaction of selenosulfonate with isocyanides and allyl alcohol under aqueous conditions to afford selenocarbamates and allyl sulfone compounds. The sulfinic acid as the first step side product is converted to the allyl sulfone compound by water promoteed reaction with allyl alcohol. Water acts as both an oxygen source of selenocarbamates and as a promoter to drive the second step reaction. The reactions have the advantages of mild conditions, green, environment-friendly, and high atomic economy.
Declaration of competing interestThe authors declare no completing financial interest.
AcknowledgmentsWe gratefully acknowledge the National Natural Science Foundation of China (Nos. 21971174, 21772137, 21672157), the Ph.D. Programs Foundation of PAPD, the Project of Scientific and Technologic Infrastructure of Suzhou (No. SZS201708), the Major Basic Research Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions (No. 16KJA150002), Soochow University, and State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials for financial support.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.07.007.
| [1] |
(a) C.W. Nogueira, G. Zeni, J.B.T. Rocha, Chem. Rev. 104 (2004) 6255-6285; (b) A.D. Giuseppe, R. Castarlenas, J.J. Pérez-Torrenté, et al., J. Am. Chem. Soc.134 (2012) 8171-8183; (c) A. Ferry, T. Billard, B.R. Langlois, E. Bacqu, Angew. Chem. Int. Ed. 48 (2009) 8551-8555; (d) G. Danoun, B. Bayarmagnai, M.F. Gruenberg, L.J. Goossen, Chem. Sci. 5 (2014) 1312-1316; (e) C.P. Zhang, D.A. Vicic, J. Am. Chem. Soc. 134 (2012) 183-185; (f) D. Yang, K. Yan, W. Wei, et al., J. Org. Chem. 80 (2015) 6083-6092; (g) G. Teverovskiy, D.S. Surry, S.L. Buchwald, Angew. Chem. Int. Ed. 50 (2011) 7312-7314. |
| [2] |
(a) P.C. Srivastava, R.K. Robins, J. Med. Chem. 26 (1983) 445-448; (b) G. Mugesh, W.W. Mont, H. Sies, Chem. Rev. 101 (2001) 2125-2179; (c) A. Heredia, A.B. Peñéñory, J. Org. Chem. 13 (2017) 910-918; (d) Y. Li, M. Wang, X. Jiang, ACS Catal. 7 (2017) 7587-7592; (e) E.A. Ilardi, E. Vitaku, J.T. Njardarson, J. Med. Chem. 57 (2014) 2832-2842; (f) Y. Li, M. Wang, X. Jiang, ACS Catal. 7 (2017) 7587-7592. |
| [3] |
H. Takahashia, A. Nishinaa, R. Fukumotoa, et al., Eur. J. Pharm. Sci. 24 (2005) 291-295. DOI:10.1016/j.ejps.2004.11.004 |
| [4] |
(a) B.K. Sarma, D. Manna, M. Minoura, G. Mugesh, J. Am. Chem. Soc. 132 (2010) 5364-5374; (b) B.K. Sarma, G. Mugesh, Chem. Eur. J. 14 (2008) 10603-10614. |
| [5] |
K. Takimiya, I. Osaka, T. Mori, M. Nakano, Acc. Chem. Res. 47 (2014) 1493-1502. DOI:10.1021/ar400282g |
| [6] |
(a) Y. Fang, T. Rogge, L. Ackermann, S.Y. Wang, S.J. Ji, Nat. Comm. 9 (2018) 2240; (b) H. Liu, Y. Fang, L. Yin, S.Y. Wang, S.J. Ji, J. Org. Chem. 82 (2017) 10866-10874; (c) H. Liu, Y. Fang, S.Y. Wang, S.J. Ji, Org. Lett. 20 (2018) 930-933; (d) C. Liu, Y. Fang, S.Y. Wang, S.J. Ji, Org. Lett. 20 (2018) 6112-6116. |
| [7] |
(a) J.H. Li, Q. Huang, W. Rao, S.Y. Wang, S.J. Ji, Chem. Commun. 55 (2019) 7808-7811; (b) J.H. Li, Q. Huang, S.Y. Wang, S.J. Ji, Org. Lett. 20 (2018) 4704-4708; (c) B.B. Liu, X.Q. Chu, H. Liu, et al., J. Org. Chem. 82 (2017) 10174-10180. |
| [8] |
S. Fujiwara, K. Okada, Y. Shikano, et al., J. Org. Chem. 72 (2007) 273-276. DOI:10.1021/jo0615908 |
| [9] |
J. Xu, R.Y. Liu, C.S. Yeung, S.L. Buchwald, ACS Catal. 9 (2019) 6461-6466. DOI:10.1021/acscatal.9b01913 |
| [10] |
(a) X. Ma, S.B. Herzon, Beilstein, J. Org. Chem. 14 (2018) 2259-2265; (b) A.P. Schaffner, F. Montermini, D. Pozzi, et al., Adv. Synth. Catal. 350 (2008) 1163-1167; (c) X. Ma, S.B. Herzon, Chem. Sci. 6 (2015) 6250-6255; (d) P. Mampuys, Y. Zhu, T. Vlaar, et al., Angew. Chem. Int. Ed. 53 (2014) 12849-12854; (e) J. Li, D. Zhu, L. Lv, C.J. Li, Chem. Sci. 9 (2018) 5781-5786; (f) W. Kong, C. Yu, H. An, Q. Song, Org. Lett. 20 (2018) 4975-4978; (g) B. Xu, D. Li, L. Lu, et al., Org. Chem. Front. 5 (2018) 2163-2166. |
| [11] |
X. Peng, C. Ma, C.H. Tung, Z. Xu, Org. Lett. 18 (2016) 4154-4157. DOI:10.1021/acs.orglett.6b02027 |
| [12] |
Y. Fang, C. Liu, F. Wang, et al., Org. Chem. Front. 6 (2019) 660-663. |
| [13] |
(a) S. Oida, Y. Tajima, T. Konosu, et al., Chem. Pharm. Bull. 48 (2000) 694-707; (b) N.A. McGrath, M. Brichacek, J.T. Njardarson, J. Chem, Educ. 87 (2010) 1348-1349; (c) A.B. Pritzius, B. Breit, Angew. Chem. Int. Ed. 54 (2015) 3121-3125. |
| [14] |
M. Lee, M. Ikejiri, D. Klimpel, et al., ACS Med. Chem. Lett. 3 (2012) 490-495. DOI:10.1021/ml300050b |
| [15] |
(a) T. Song, H. Li, F. Wei, C.H. Tung, Z. Xu, Tetrahedron Lett. 60 (2019) 916-919; (b) H. Qian, K. Sulfones, X. Huang, Synthesis 12 (2006) 1934-1936; (c) S. Huang, Z. Chen, H. Mao, et al., Org. Biomol. Chem. 17 (2019) 1121-1129; (d) R.A. Gancarz, J.L. Kice, J. Org. Chem. 46 (1981) 4899-4906; (e) C. Rao, S. Maia, Q. Song, Chem. Commun. 54 (2018) 5964-5967. |
| [16] |
Y.L. Shi, M. Shi, Org. Biomol. Chem. 3 (2005) 1620-1621. DOI:10.1039/b501942g |
| [17] |
S. Huang, N. Thirupathi, C.H. Tung, Z. Xu, J. Org, Chem 83 (2018) 9449-9455. |
| [18] |
Z. Yuan, H.Y. Wang, X. Mu, et al., J. Am. Chem. Soc. 137 (2015) 2468-2471. DOI:10.1021/ja5131676 |
| [19] |
P. Xie, J. Wang, Y. Liu, et al., Nat. Commun. 9 (2018) 1321. DOI:10.1038/s41467-018-03698-8 |
 2021, Vol. 32
2021, Vol. 32