b Hunan University of Medicine, Huaihua 418000, China
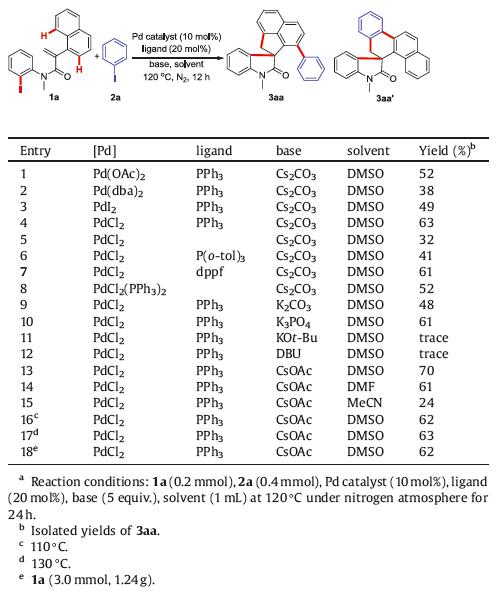
Transition-metal-catalyzed C-H functionalization of arenes has emerged as a powerful tool for the assembly of target molecules due to their high atom economy [1, 2], Generally, the directing groups need to be installed on the arenes, which assist transition-metal catalysts to accomplish the site-selective C-H bond activation [2]. However, a major issue is that additional steps are required to achieve the pre-installation and removal of directing groups. Alternatively, a palladium-catalyzed domino process is employed to achieve C-H functionalization of arenes [3-5], which is very interesting to synthetic organic chemists because they enable the formation of multiple chemical bonds in an efficient, atom- and step-economical manners in one pot. In this field, palladium-catalyzed domino reaction of alkene-tethered aryl halides has attracted tremendous attention [5-12]. This procedure discovered initially by Grigg and co-workers [6] undergoes an intramolecular Heck reaction and C–H bond activation sequence to generate the key intermediate palladacycle, which can be converted into various polycyclic compounds by three different types of strategies, such as direct reductive elimination [7], [1,4]-Pd shift [8], and reaction with a variety of coupling partners [9-11] (e. g., diaziridinones, α-diazocarbonyl compounds, disilanes, o-bromobenzoic acids, dibromomethane, aryl and alkyl iodides, arynes, and activated alkynes). Undoubtedly, the third strategy could increase the resulting product diversity by introducing coupling partners, which is more attractive than the first two conversions where the inherent C-H bond was served as the terminated functional group.
Naphthalenes as the simplest fused arenes, whose site-selective C-H functionalization have been widely studied based on the presence of C1-directing groups [12-15]. However, the vast majority of methods have focused on single C2-H [12], C3-H [13], and C8-H activation [14] of naphthalenes under transition-metal catalysis (Scheme 1A-a). In contrast, the reports on their double C-H functionalization are relatively rare. Despite the fact that the You group has recently demonstrated a palladium-catalyzed regioselective C7-H and C8-H arylations of 1-naphthamides for the construction of carbohelicenes via a directing group-assisted strategy (Scheme 1A-b) [15], the development of new efficient methods for double C-H activation of naphthalenes is urgently needed and extremely intriguing. Our group reported a series of palladium-catalyzed tandem reactions of aryl iodides involving C(aryl)-H activation in recent years [11, 16]. In continuation of our interest in this field, we envision that double C-H functionalization of naphthalene ring could be achieved by the domino reaction of N-(2-iodophenyl)-2-(naphthalen-1-yl)acrylamides with aryl iodides. Herein, we present a new transformation between N-(2-iodophenyl)-2-(naphthalen-1-yl)acrylamides and aryl iodides for the synthesis of aryl-substituted spirocyclic oxindoles, which allows three C-C bonds formation by sequential C2-H arylation and C8-H alkylation of naphthalene ring (Scheme 1B).
|
Download:
|
| Scheme1. Two different strategies for transition-metal-catalyzed C—H functionalization of naphthalene ring. | |
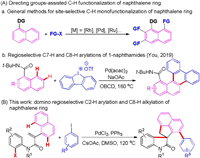
We began our investigation with the tandem reaction of N-(2-iodophenyl)- N-methyl-2-(naphthalen-1-yl)acrylamide 1a with iodobenzene 2a (Table 1). Unexpectedly, the product 3aa, rather than 3aa′, was obtained exclusively in 52% yield via sequentially selective C-H activation under the reaction conditions reported by our previous work (entry 1). Inspired by this intriguing result, a scrupulous screening of various reaction parameters was performed. Evaluation of palladium catalysts (Pd(dba)2, PdI2, and PdCl2) and P-ligands (P(o-tol)3 and dppf) showed that a combination of PdCl2 with PPh3 was the best choice (entries 2–7). Unfortunately, replacing PdCl2 and PPh3 with PdCl2(PPh3)2 displayed lower catalytic efficiency (entry 8). Subsequently, CsOAc was found to be the most competent base in terms of yield (entries 9–13). Finally, Inferior results were afforded by performing the reaction with other solvents (DMF and MeCN) or reaction temperature (110 ℃ and 130 ℃) (entries 14–17). Accordingly, the optimal reaction conditions were shown as entry 13 in Table 1. Gratifyingly, a gram-scale experiment of 1a (3 mmol, 1.24 g) with iodobenzene 2a could afford the product 3aa in 62% yield (entry 18).
|
|
Table 1 Optimization of the reaction conditions. |
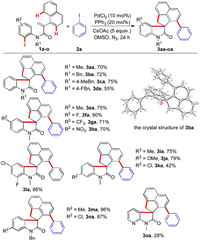
Having the optimized reaction conditions in hand, we set out to examine the substrate scope of this cascade reaction. A variety of substituted N-(2-iodophenyl)-2-(naphthalen-1-yl)acrylamides 1 were initially investigated (Scheme 2). Acrylamides bearing different substituents on the nitrogen atom could undergo this cyclization/cross coupling cascade to produce the target products 3ba-3da in moderated to good yields. The structure of the product 3ba was clearly verified by single-crystal X-ray analysis. Delightedly, substitution with electron-withdrawing (F, Cl, CF3 and NO2) as well as electron-donating groups (Me and OMe) on the aromatic ring of the aniline moiety were well tolerated under the standard conditions, and the desired products 3ea-3na were afforded in 42%–90% yields. It was noteworthy that acrylamides 1o from heterocyclic arylamine could be smoothly converted into the product 3oa, albeit only with a 28% yield.
|
Download:
|
| Scheme 2. Scope of the N-(2-iodophenyl)-2-(naphthalen-1-yl)acrylamides. Reaction conditions: 1 (0.2 mmol), 2a (0.4 mmol), PdCl2 (10 mol%), PPh3 (20 mol%), CsOAc (5 equiv.), DMSO (1 mL) at 120 ℃ under nitrogen atmosphere for 24 h. | |
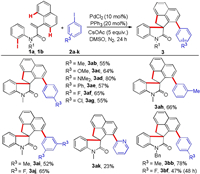
The substrate scope of aryl iodides 2 was explored next, as demonstrated in Scheme 3. We were pleased to found that monosubstituted aryl iodides possessing Me, OMe, NMe2, Ph, F and Cl groups at the para or meta position could be efficiently coupled producing the aryl-substituted spirocyclic oxindoles 3ab-3ah, 3bb, and 3bf in moderate to good yields. Unfortunately, ortho-substituted aryl iodides, such as 1-iodo-2-methylbenzene and 1-fluoro-2-iodobenzene, could not be converted into the desired products. The results demonstrated that the cascade reaction was dramatically affected by the steric effect of the substituents on aryl iodides. Disubstituted aryl iodides (2i and 2j) proved to be also amenable substrates, producing 3ai and 3aj in 52% and 65% yields, respectively. However, the reaction of 2-iodopyridine 2k with acrylamides delivered the product 3ak in a lower yield in comparison with other aryl iodides.
|
Download:
|
| Scheme 3. Scope of the aryl iodides. Reaction conditions: entry 13 in Table 1 and Scheme 2. | |
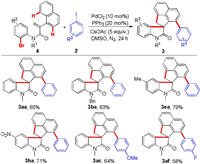
Finally, the generality of this protocol was further examined by using N-(2-bromophenyl)-2-(naphthalen-1-yl)acrylamides and aryl iodides as the reaction substrates (Scheme 4). Gratifyingly, the reaction of N-(2-bromophenyl)acrylamide 4a with iodobenzene could afford 65% of the target product 3aa under the standard conditions. Subsequently, several N-(2-bromophenyl)acrylamide (4b, 4e, and 4h) and aryl iodides (1c and 1f) were investigated. All of them were subjected to the cascade cyclization to provide the desired products (3ba, 3ea, 3ha, 3ac, and 3af) in 63%–79% yield.
|
Download:
|
| Scheme 4. The reaction of N-(2-bromophenyl)-2-(naphthalen-1-yl)acrylamides with aryl iodides. Reaction conditions: entry 13 in Table 1 and Scheme 2. | |
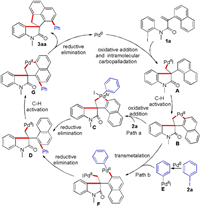
On the basis of Lautens's work [10e] and our previous report [11c], a possible mechanism for this palladium-catalyzed cascade reaction is depicted in Scheme 5. First, oxidative addition of N-(2-iodophenyl)acrylamide 1a to Pd(0) species followed by an intramolecular Heck reaction forms the intermediate A. Next, the C2-H activation of the aryl Pd(II) species A to the naphthalene ring produces the spiropalladacycle B, which inserts into the C-I bond of iodobenzene 2a via oxidative addition to give spiropallada(IV)cycle C. Intermediate D was then formed by the reductive elimination of intermediate C. Finally, intermediate D undergoes the second C-H activation of naphthalene ring followed by reductive elimination to give product 3aa and regenerate Pd(0) (path a). Furthermore, the transmetelation pathway of spiropalladacycle B with intermediate E generated from iodobenzene 2a cannot be ruled out (path b).
|
Download:
|
| Scheme 5. Possible reaction mechanism. | |
In summary, we have disclosed a new palladium-catalyzed cascade reaction for the assembly of a variety of aryl-substituted spirocyclic oxindoles from N-(2-halophenyl)-2-(naphthalen-1-yl)acrylamides with aryl iodides, which enables regioselective C2-H arylation and C8-H alkylation of naphthalene ring through an intramolecular carbopalladation/twice C-H activation sequence. Notably, this approach showcases broad substrate scope and good functional group tolerance. Further studies toward exploring this type of cascade reaction are still in progress in our laboratory.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank the National Natural Science Foundation of China (Nos. 21572051, 21602057, 21901071, and 21971061), Natural Science Foundation of Hunan Province (Nos. 2020JJ5350 and 2020JJ5347), Scientific Research Fund of Hunan Provincial Education Department (Nos. 18A002, 19B359 and 17C1137), and Science and Technology Planning Project of Hunan Province (No. 2018TP1017) for financial support.
Appendix A.Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.07.005.
| [1] |
(a) X.S. Xue, P. Ji, B. Zhou, J.P. Cheng, Chem. Rev. 117 (2017) 8622-8648; (b) C. Liu, J. Yuan, M. Gao, et al., Chem. Rev. 115 (2015) 12138-12204; (c) D. Alberico, M.E. Scott, M. Lautens, Chem. Rev. 107 (2007) 174-238. |
| [2] |
(a) J. He, M. Wasa, K.L. Chan, Q. Shao, J.Q. Yu, Chem. Rev. 117 (2017) 8754-8786; (b) T. Gensch, M.N. Hopkinson, F. Glorius, J. Wencel-Delord, Chem. Soc. Rev. 45 (2016) 2900-2936; (c) H. Huang, X. Ji, W. Wu, H. Jiang, Chem. Soc. Rev. 44 (2015) 1155-1171; (d) Z. Huang, H.N. Lim, F. Mo, M.C. Young, G. Dong, Chem. Soc. Rev. 44 (2015) 7764-7786; (e) K.M. Engle, T.S. Mei, M. Wasa, J.Q. Yu, Acc. Chem. Res. 45 (2012) 788-802; (f) S.R. Neufeldt, M.S. Sanford, Acc. Chem. Res. 45 (2012) 936-946. |
| [3] |
M. Catellani, F. Frignani, A. Rangoni, Angew. Chem. Int. Ed. 36 (1997) 119-122. |
| [4] |
(a) J. Wang, G. Dong, Chem. Rev. 119 (2019) 7478-7528; (b) H.G. Cheng, S. Chen, R. Chen, Q. Zhou, Angew. Chem. Int. Ed. 58 (2019) 5832-5844; (c) Z.S. Liu, Q. Gao, H.G. Cheng, Q. Zhou, Chem. Eur. J. 24 (2018) 15461-15476; (d) D.S. Kim, W.J. Park, C.H. Jun, Chem. Rev. 117 (2017) 8977-9015; (e) N. Della Ca', M. Fontana, E. Motti, M. Catellani, Acc. Chem. Res. 49 (2016) 1389-1400; (f) J. Ye, M. Lautens, Nat. Chem. 7 (2015) 863-870. |
| [5] |
(a) Y. Ping, Y. Li, J. Zhu, W. Kong, Angew. Chem. Int. Ed. 58 (2019) 1562-1573; (b) I. Franzoni, H. Yoon, J.A. García-López, A.I. Poblador-Bahamonde, M. Lautens, Chem. Sci. 9 (2018) 1496-1509; (c) V.P. Mehta, J.A. García-López, ChemCatChem 9 (2017) 1149-1156; (d) M. Pe'rez-Go'mez, L. Navarro, I. Saura-Llamas, et al., Organometallics 36 (2017) 4465-4476. |
| [6] |
(a) D. Brown, K. Grigg, V. Sridharan, V. Tambyrajah, Tetrahedron Lett. 44 (1995) 8137-8140; (b) R. Grigg, P. Fretwell, C. Meerholtz, V. Sridharan, Tetrahedron 50 (1994) 359-370. |
| [7] |
(a) N. Saha, H. Wang, S. Zhang, et al., Org. Lett. 20 (2018) 712-715; (b) Y.C. Wu, S.S. Jiang, R.J. Song, J.H. Li, Chem. Commun. (Camb. ) 55 (2019) 4371-4374; (c) T. Piou, L. Neuville, J. Zhu, Angew. Chem. Int. Ed. 51 (2012) 11561-11565; (d) T. Piou, L. Neuville, J. Zhu, Org. Lett. 14 (2012) 3760-3763; (e) R.T. Ruck, M.A. Huffman, M.M. Kim, et al., Angew. Chem. Int. Ed. 47 (2008) 4711-4714. |
| [8] |
(a) Z.Y. Gu, C.G. Liu, S.Y. Wang, S.J. Ji, Org. Lett. 18 (2016) 2379-2382; (b) A. Bunescu, T. Piou, Q. Wang, J. Zhu, Org. Lett. 17 (2015) 334-337; (c) T. Piou, A. Bunescu, Q. Wang, L. Neuville, J. Zhu, Angew. Chem. Int. Ed. 52 (2013) 12385-12389; (d) Z. Lu, C. Hu, J. Guo, et al., Org. Lett. 12 (2010) 480-483; (e) Q. Huang, A. Fazio, G. Dai, M.A. Campo, R.C. Larock, J. Am. Chem. Soc. 126 (2004) 7460-7461. |
| [9] |
(a) J.G. Liu, W.W. Chen, C.X. Gu, B. Xu, M.H. Xu, Org. Lett. 20 (2018) 2728-2732; (b) A. Lu, X. Ji, B. Zhou, Z. Wu, Y. Zhang, Angew. Chem. Int. Ed. 57 (2018) 3233-3237; (c) C. Shao, Z. Wu, X. Ji, B. Zhou, Y. Zhang, Chem. Commun. (Camb. ) 53 (2017) 10429-10432; (d) M. Pérez-Gómez, S. Hernández-Ponte, D. Bautistab, J.A. García-López, Chem. Commun. (Camb. ) 53 (2017) 2842-2845; (e) M. Péreⱬ-Gómez, J.A. García-Lópeⱬ, Angew. Chem. Int. Ed. 55 (2016) 14389-14393; (f) T. Yao, D. He, Org. Lett. 19 (2017) 842-845; (g) H. Zheng, Y. Zhu, Y. Shi, Angew. Chem. Int. Ed. 53 (2014) 11280-11284. |
| [10] |
(a) J.F. Rodríguez, A.D. Marchese, M. Lautens, Org. Lett. 20 (2018) 4367-4370; (b) J. Ye, Z. Shi, T. Sperger, et al., Nat. Chem. 9 (2017) 361-368; (c) H. Yoon, M. Rölz, F. Landau, M. Lautens, Angew. Chem. Int. Ed. 56 (2017) 10920-10923; (d) H. Yoon, A. Lossouarn, F. Landau, M. Lautens, Org. Lett. 18 (2016) 6324-6327; (e) M. Sickert, H. Weinstabl, B. Peters, X. Hou, M. Lautens, Angew. Chem. Int. Ed. 53 (2014) 5147-5251. |
| [11] |
(a) X. Yang, H. Lu, X. Zhu, et al., Org. Lett. 21 (2019) 7284-7288; (b) X. Luo, L. Zhou, H. Lu, et al., Org. Lett. 21 (2019) 9960-9964; (c) X. Luo, Y. Xu, G. Xiao, et al., Org. Lett. 20 (2018) 2997-3000. |
| [12] |
(a) G. Tan, Q. You, J. Lan, J. You, Angew. Chem. Int. Ed. 57 (2018) 6309-6313; (b) G. Tan, Q. You, J. You, ACS Catal. 8 (2018) 8709-8714; (c) H. Miura, S. Terajima, T. Shishido, ACS Catal. 8 (2018) 6246-6254; (d) Y.C. Wu, S.S. Jiang, S.Z. Luo, R.J. Song, J.H. Li, Chem. Commun. (Camb. ) 55 (2019) 8995-8998; (e) S.L. Liu, X.H. Li, S.S. Zhang, et al., Adv. Synth. Catal. 359 (2017) 2241-2246; (f) Y. Zhang, H. Zhao, M. Zhang, W. Su, Angew. Chem. Int. Ed. 54 (2015) 3817-3821; (g) F.J. Chen, G. Liao, X. Li, J. Wu, B.F. Shi, Org. Lett. 16 (2014) 5644-5647; (h) J. Ryu, J. Kwak, K. Shin, D. Lee, S. Chang, J. Am. Chem. Soc. 135 (2013) 12861-12868. |
| [13] |
(a) P. Wang, M.E. Farmer, X. Huo, et al., J. Am. Chem. Soc. 138 (2016) 9269-9676; (b) P. Wang, G.C. Li, P. Jain, et al., J. Am. Chem. Soc. 138 (2016) 14092-14099; (c) X.C. Wang, W. Gong, L.Z. Fang, et al., Nature 519 (2015) 334-338. |
| [14] |
(a) T. Fukuyama, T. Sugimori, S. Maetani, I. Ryu, Org. Biomol. Chem. 16 (2018) 7583-7587; (b) D. Sun, B. Li, J. Lan, Q. Huang, J. You, Chem. Commun. (Camb. ) 52 (2016) 3635-3638; (c) Z.W. Yang, Q. Zhang, Y.Y. Jiang, et al., Chem. Commun. (Camb. ) 52 (2016) 6709-6711; (d) N. Luo, Z. Yu, Chem. Eur. J. 16 (2010) 787-791; (e) K.L. Hull, M.S. Sanford, J. Am. Chem. Soc. 129 (2007) 11904-11905. |
| [15] |
M. Wang, M. Zhang, Y. Luo, et al., Org. Lett. 22 (2020) 135-139. |
| [16] |
(a) W. Li, W. Chen, B. Zhou, et al., Org. Lett. 21 (2019) 2718-2722; (b) Y. Xu, X. Liu, W. Chen, et al., J. Org. Chem. 83 (2018) 13930-13939; (c) Y. Yang, B. Zhou, X. Zhu, et al., Org. Lett. 20 (2018) 5402-5405; (d) G. Xiao, L. Chen, B. Zhou, et al., Adv. Synth. Catal. 360 (2018) 3477-3481; (e) L. Wu, G. Deng, Y. Liang, Org. Biomol. Chem. 15 (2017) 6808-6812; (f) G. Xiao, L. Chen, G. Deng, J. Liu, Y. Liang, Tetrahedron Lett. 59 (2018) 1836-1840; (g) W. Li, G. Xiao, G. Deng, Y. Liang, Org. Chem. Front. 5 (2018) 1488-1492. |
 2021, Vol. 32
2021, Vol. 32