b State Key Laboratory of Bioorganic and Natural Products Chemistry, Center for Excellence in Molecular Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200032, China
Native protein modification plays a crucial role in the development of therapeutic biologics, such as PEGylated proteins, antibody−drug conjugates, and glycoconjugate vaccines [1]. The thiol within a cysteine residual and the primary amine within a lysine residual or the N-terminus serve as convenient handles for chemical modification of native proteins, because of their superior nucleophilicity [2]. However, covalent modification of the thiol may interfere disulfide formation that is crucial for protein folding. Thus, methods for labeling the primary amine have been increasingly attractive for native protein modification. The conventional strategy that relies on acylating reagents such as the N-hydroxysuccinimide (NHS) ester [3] often suffer reactivity, chemoselectivity, and purification issues. In 1909, Thiele and Schneider reported that ortho-phthalaldehyde (OPA) reacted with primary amines under mild conditions to afford the corresponding N-substituted isoindolinones [4]. A recent computational study by Alajarín et al. suggested that the transformation should proceed through a sequence of intermolecular imine formation, [1,5]-H shift, and cyclization [5]. The Thiele reaction had long been underappreciated from a synthesis perspective, until Li and co-workers exploited it to develop an elegant approach for traceless and chemoselective amine bioconjugation in 2016 (Fig. 1) [6]. They showcased the power of this method with immobilization of red fluorescent protein (RFP) and construction of PEGlyated asparaginase [6]. Very recently, Li and colleagues developed an OPA−amine−thiol three-component reaction for chemoselective peptide cyclization [7], which broadens the use of OPA reagents in peptide chemistry. Li's synthesis of OPA reagents featured a Pb(OAc)4 mediated oxidative formylation reaction. Of note, 5-substituted salicylaldehydes needed to be employed as starting materials, and bis-acetal protection of the OPA intermediates was required prior to side chain functionalization [6]. Our experience with aromatic natural product synthesis [8, 9] suggested an opportunity for expeditious preparation of Li−Thiele reagents based on an arene construction strategy [8]. Herein, we report a short and scalable route for synthesis of an alkyne-containing Li−Thiele reagent.

|
Download:
|
| Fig. 1. Immobilization of red fluorescent protein using a Li—Thiele reagent. | |
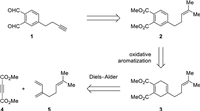
We envisioned OPA derivative 1 (Fig. 2) as a bifunctional Li−Thiele reagent; the terminal alkyne could serve as a handle for secondary modification through copper catalyzed azide−alkyne cycloaddition (CuAAC) [10]. The structural features of 1 suggested that an arene construction approach [8] should be considerably more efficient than a conventional arene modification approach. From a retrosynthetic perspective, 1 was traced back to phthalate 2, which might arise from 1, 4-cyclohexadiene derivative 3 (Fig. 2). Oxidative aromatization of 3 was expected to be effected by exposure to O2 [8a–f] or DDQ [8g, h, k]. Compound 3 could be assembled by an intermolecular Diels−Alder reaction of readily available dimethyl acetylenedicarboxylate (4) and β-myrcene (5). Of note, a similar Diels−Alder/aromatization sequence was well-precedented in the literature [11].
|
Download:
|
| Fig. 2. Retrosynthetic analysis of a bifunctional Li—Thiele reagent. | |
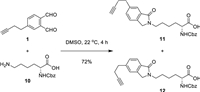
Our synthesis of 1 was illustrated in Scheme 1. Diels−Alder reaction of 4 and 5 proceeded smoothly at 80℃ to afford cycloadduct 3, which, upon treatment with DDQ, underwent oxidative aromatization to furnish phthalate 2 in 83% overall yield [11a]. Ozonolysis of the trisubstituted olefin gave aldehyde 6, and Seyferth−Gilbert homologation [12] with Ohira−Bestmann reagent [13] (7) in basic MeOH provided terminal alkyne 8 with good efficiency. Reduction of 8 with LiAlH4 afforded diol 9 in 79% yield. Conversion of this diol into bis-aldehyde 1 proved to be nontrivial. The partial oxidation product γ-hydroxyaldehyde underwent instantaneous cyclization, and the resultant lactol was prone to oxidation leading to the corresponding lactone. Therefore, we needed to prevent the free hydroxy group from meeting with the aldehyde carbonyl group during the course of oxidation. Swern oxidation was expected to address this issue because of the particular mode of alcohol activation [14]. To our delight, 1 was obtained in 88% yield under standard Swern conditions [DMSO, (COCl)2, Et3N]. Of note, this synthesis was reliably preformed on a gram scale. We further examined the reaction of 1 with N-α-Cbz-L-lysine (10) (Scheme 2). As expected, Thiele reaction proceeded smoothly to give a mixture of two regioisomers 11 and 12 (ca. 1.2:1 ratio). The modest regioselectivity is inconsequential from a ligation perspective.

|
Download:
|
| Scheme 1. Gram-scale preparation of 1. | |
|
Download:
|
| Scheme 2. Reaction of N-α-Cbz-L-lysine (10) with reagent 1. | |
In summary, we developed an expeditious and scalable approach for preparation of Li−Thiele reagent 1, taking advantage of an arene construction strategy. This reagent may find use in amine-based bioconjugation.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis paper is dedicated to Prof. Henry N.C. Wong. We thank Prof. Xuechen Li and Xiang Zhang for discussion. This work was supported by Ministry of Science and Technology (National Key Research and Development Program of China, No. 2018YFA0901900), the National Natural Science Foundation of China (Nos. 21931014, 21525209, 21621002, 21772225, and 21761142003), Chinese Academy of Sciences (Strategic Priority Research Program, No. XDB20000000; International Partner Program, No. 121731KYSB20190039; Key Research Program of Frontier Sciences, No. QYZDB-SSW-SLH040), Science and Technology Commission of Shanghai Municipality (No. 17XD1404600), and K.C. Wong Education Foundation.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.06.019.
| [1] |
(a) Y. Takaoka, A. Ojida, I. Hamachi, Angew. Chem. Int. Ed. 52 (2013) 4088-4106; (b) O. Boutureira, G.J.L. Bernardes, Chem. Rev. 115 (2015) 2174-2195; (c) E.A. Hoyt, P.M.S.D. Cal, B.L. Oliveira, G.J.L. Bernardes, Nat. Rev. Chem. 3 (2019) 147-171. |
| [2] |
O. Koniev, A. Wagner, Chem. Soc. Rev. 44 (2015) 5495-5551. DOI:10.1039/C5CS00048C |
| [3] |
A.J. Lomant, G. Fairbanks, J. Mol. Biol. 104 (1976) 243-261. |
| [4] |
J. Thiele, J. Schneider, Justus Liebigs Ann. Chem. 369 (1909) 287-299. DOI:10.1002/jlac.19093690304 |
| [5] |
M. Alajarín, P. Sánchez-Andrada, C. López-Leonardo, M. A.Álvarez, J. Org. Chem. 70 (2005) 7617-7623. DOI:10.1021/jo0508494 |
| [6] |
C.L. Tung, C.T. Wong, E.Y.M. Fung, X. Li, Org. Lett. 18 (2016) 2600-2603. DOI:10.1021/acs.orglett.6b00983 |
| [7] |
Y. Zhang, Q. Zhang, C.T.T. Wong, X. Li, J. Am. Chem. Soc. 141 (2019) 12274-12279. DOI:10.1021/jacs.9b03623 |
| [8] |
(a) Z. Lu, Y. Li, J. Deng, A. Li, Nat. Chem. 5 (2013) 679-684; (b) J. Li, P. Yang, M. Yao, J. Deng, A. Li, J. Am. Chem. Soc. 136 (2014) 16477-16480; (c) Z. Meng, H. Yu, L. Li, et al., Nat. Commun. 6 (2015) 6096; (d) M. Yang, J. Li, A. Li, Nat. Commun. 6 (2015) 6445; (e) M. Wan, M. Yao, J.Y. Gong, et al., Chin. Chem. Lett. 26 (2015) 272-276; (f) M. Yang, X. Yang, H. Sun, A. Li, Angew. Chem. Int. Ed. 55 (2016) 2851-2855; (g) P. Yang, M. Yao, J. Li, Y. Li, A. Li, Angew. Chem. Int. Ed. 55 (2016) 6964-6968; (h) H. Li, Q. Chen, Z. Lu, A. Li, J. Am. Chem. Soc. 138 (2016) 15555-15558; (i) Z. Zhang, J. Wang, J. Li, et al., J. Am. Chem. Soc. 139 (2017) 5558-5567; (j) Y. Chen, W. Zhang, L. Ren, J. Li, A. Li, Angew. Chem. Int. Ed. 57 (2018) 952-956; (k) P. Yang, J. Li, L. Sun, et al., J. Am. Chem. Soc. 142 (2020) 13701-13708; (l) Z. Lu, M. Yang, P. Chen, X. Xiong, A. Li, Angew. Chem. Int. Ed. 53 (2014) 13840-13844. |
| [9] |
(a) J. Deng, R. Li, Y. Luo, et al., Org. Lett. 15 (2013) 2022-2025; (b) J. Deng, S. Zhou, W. Zhang, et al., J. Am. Chem. Soc. 136 (2014) 8185-8188; (c) S. Zhou, H. Chen, Y. Luo, W. Zhang, A. Li, Angew. Chem. Int. Ed. 54 (2015) 6878-6882; (d) S. Zhou, R. Guo, P. Yang, A. Li, J. Am. Chem. Soc. 140 (2018) 9025-9029. |
| [10] |
(a) H.C. Kolb, M.G. Finn, K.B. Sharpless, Angew. Chem. Int. Ed. 40 (2001) 2004-2021; (b) H.C. Kolb, K.B. Sharpless, Drug Discov. Today 8 (2003) 1128-1137. |
| [11] |
(a) G.S. Heo, B. Moon, Tetrahedron Lett. 49 (2008) 5540-5543; (b) M. Deng, Y. Yao, X. Li, et al., Org. Lett. 21 (2019) 3290-3294. |
| [12] |
(a) J.D. Seyferth, R.S. Marmor, P. Hilbert, J. Org. Chem. 36 (1971) 1379-1386; (b) J.C. Gilbert, U. Weerasooriya, J. Org. Chem. 47 (1982) 1837-1845. |
| [13] |
(a) S. Ohira, Synth. Commun. 19 (1986) 561-564; (b) S. Müller, B. Liepold, G.J. Roth, H.J. Bestmann, Synlett 1996 (1996) 521-522. |
| [14] |
O. Farooq, Synthesis 1994 (1994) 1035-1036. DOI:10.1055/s-1994-25632 |
 2021, Vol. 32
2021, Vol. 32 

