b State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, China;
c Department of Chemistry and Materials Science, College of Science, Nanjing Forestry University, Nanjing 210037, China
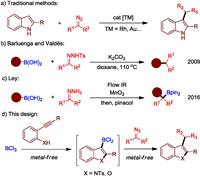
The indole unit exists ubiquitously in biologically active molecules, natural products, pharmaceuticals, and agrochemicals [1]. Therefore, the development of new methods for the synthesis of functionalized indole derivatives has been a topic of great importance [2]. C3-Substituted indoles are the core structure of various drugs and medicinally important agents [3], transitionmetal-catalyzed functionalization of indoles by carbene transfer provides an attractive route to produce these compounds (Fig. 1a). Many groups have achieved the C-H functionalization of indoles with diazo compounds to build C−C bonds at C3-position [4]. More recently, other transition-metals like Au [5], Pd [6], Cu [7], Fe [8] and Mb [9] have also been employed to enable these transformations. Due to economic and toxicity concerns of transition-metals, there is a significant interest in the development of metal-free variant to mimic such conversions.
|
Download:
|
| Fig. 1. Reaction design. | |
The generation of C-C bonds is at the heart of synthetic organic chemistry and the development of new method to build carbon–carbon bonds under metal-free conditions is attracting increasing attention [10]. In 2010, Barluenga and Valdés first developed an efficient method on C-C bond couplings between tosylhydrazones and boronic acids without any transition metals and catalysts (Fig. 1b) [11]. In 2016, Ley group also uncovered the in situ preparation of reactive allylic and benzylic boronic acids, obtained by reacting flow-generated diazo compounds with boronic acids, and their application in controlled iterative C-C bond forming reactions (Fig. 1c) [12]. Boron halides such as BCl3 and BBr3, are attractive borylation agents because they are commercially available in multigram to kilogram quantities and are cheaper than most common boron reagents. In 2018, we described an efficient metal-free aminoboration of alkynes to access C3-borylated indoles with BCl3 [13]. Very recently, our group also reported a general method on construction of (hetero)aryl boronates by BBr3-mediated directed C–H borylation [14], in which one example was showcased on metal-free C7-alkylation betweenan in-situ formed 7-borylatedindole and a tosylhydrazone. Here, we report a general strategy on tandem aminoboration of alkynes and alkylation with diazo compounds to access C3-substituted indoles avoiding the use of any metals (Fig. 1d). Replacing the transition metal-catalyzed carbene transfer reactions by tandem metal-free process offers an alternative pathway for construction of substituted indoles with exciting possibilities due to its superior practicality, low cost, and environmental friendliness.
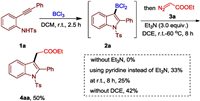
We initiated our study by investigating the cyclization of o-alkynylaniline 1a with BCl3 (Scheme 1). As a result, we discovered that the use of 1.0 equiv. of 1a with 2.0 equiv. of BCl3 in DCM at room temperature for 2.5h led to the full conversion of the precursors and formation of boron complex 2a. Then, the solvent was removed in vacuo and diazo compound 3a (3.0 equiv.) and dry Et3N (3.0 equiv.) in dry DCE was added slowly to the above system under argon atmosphere. The solution was then slowly warmed to room temperature and continued to stirr for another 2h. Subsequently the temperature increased to 60℃ slowly, and then stirred for another 6h. The desired indole product 4aa was isolated with 50% yield in this cascade reaction. When the reaction was carried out without the base, we did not observe any alkylation product. Other bases such as pyridine were also efficient for this reaction, but with much lower reactivity. Lowering the reaction temperature to room temperature led to form product 4aa in 25% yield. In addition, the use of DCM for the second step reaction was found slightly inferior than that of DCE.
|
Download:
|
| Scheme 1. Reaction development. | |
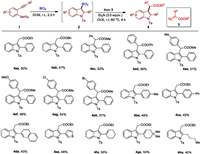
With the optimized reaction conditions in hand, we first investigated the scope of diazo compounds 3 with o-alkynylaniline 1a (Scheme 2). Diazoacetate 2a bearing a COOBn substituent also underwent facile cyclization and alkylation to generate the corresponding product 4ab in 47% yield. Diazo-phenylacetates containing COOMe (3c) and COOPr (3d) worked well under the reaction conditions, affording the corresponding products 4ac and 4ad in 52% and 56% yields. Other diazo-arylacetates including methyl (3e), methoxy (3f), Cl (3g), and Br (3h) groups at aryl motifwere compatible under the reaction conditions (4ae−4ah). Next, we turned our attention toexplore the sequential C-C bond formation using different alkynylanilines with diazo compound 3a. Aromatic groups with diverse substitution patterns like 4ba and 4ca could be transferred effectively from the corresponding o-alkynyl groups. The naphthalene and thiophene-containing products 4da-4ea were also formed in 43%–48% yields. It was noteworthy that ortho-enynyl substituted amines including 1f and 1g were tolerable with the reaction system, and the products 4fa and 4ga were isolated in 55% and 52% yields. In addition, the developed system was also tolerant of alkyl substituent, and the compound 4ha was produced in a 40% yield.
|
Download:
|
| Scheme 2. Metal-free cyclization and subsequent C—C bond formation. Reaction conditions: 1 (0.20 mmol), BCl3 (0.4 mL, 1.0 mol/L in DCM), in DCM (1.0 mL), 2.5 h, r.t., under Ar; then diazo compound 3 (0.60 mmol), dry Et3N (0.60 mmol), in dry DCE (1 mL), r.t. to 60 ℃, 8 h, under Ar. | |
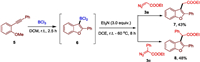
A major benefit of this metal-free and cascade strategy is that other substituted heteroaromatic compounds could also be formed. For example, C3-substituted benzofurans 7 and 8 resulted from BCl3-induced annulation oxoborationand C-C coupling with diazo compounds could be produced in 43% and 48% yields, respectively (Scheme 3) [15].
|
Download:
|
| Scheme 3. Cascade oxoboration and reaction with ethyl diazoacetate. | |
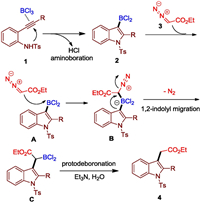
Finally, we proposed a mechanism of this tandem process in Fig. 2. Based on the previously reported results [13], the reaction of alkynylanilines 1 and BCl3 can form dichloro(indolyl)borane 2 first. Once it formed, the nucleophilic diazo carbon can attract to the BCl2 group via intermediate A to form boron species B. The 1,2-indolyl migration occurs to produce another borane species C. According to reported work [11], compound C can further convert into product 4a by protodeboronation in the presence of base and water.
|
Download:
|
| Fig. 2. Proposed mechanism. | |
In summary, we have put forward an efficient BCl3-mediated strategy for the cascade cyclization of varieties of o-alkynylanilines and C-C coupling with diazo compounds under metal-free conditions. Although the modest total yields were provided for this two-step cascade transformation, the use of this method for the preparation of substituted indoles under mild conditions avoiding the use of transition metals would still be of great importance.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis study was supported by the National Natural Science Foundation of China (Nos. 21972064 and 21672097), the Excellent Youth Foundation of Jiangsu Scientific Committee (No. BK20180007), and the "Innovation & Entrepreneurship Talents Plan" of Jiangsu Province.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.06.028.
| [1] |
(a) D.G. Batt, J.X. Qiao, D.P. Modi, et al., Biorg. Med. Chem. Lett.14 (2004) 5269-5273; (b) T. Kawasaki, K. Higuchi, Nat. Prod. Rep. 22 (2005) 761-793; (c) A.J. Kochanowska-Karamyan, M.T. Hamann, Chem. Rev. 110 (2010) 4489-4497; (d) M. Ishikura, T. Abe, T. Choshi, S. Hibin, S. Hibino, Nat. Prod. Rep. 30 (2013) 694-752; (e) A.J. Kochanowaska-Karamyan, M.T. Hamann, Chem. Rev. 110 (2010) 4489-4497; (f) S.A. Patil, R. Patil, D.D. Miller, Future Med. Chem. 4 (2012) 2085-2099; (g) N.K. Kaushik, N. Kaushik, P. Attri, et al., Molecules 18 (2013) 6620-6662; (h) M.Z. Zhang, Q. Chen, G.F. Yang, Eur. J. Med. Chem. 89 (2015) 421-441. |
| [2] |
(a) B.E. Maryano, H.C. Zhang, J.H. Cohen, I.J. Turchi, C.A. Maryano, Chem. Rev. 104 (2004) 1431-1628; (b) S. Cacchi, G. Fabrizi, Chem. Rev. 105 (2005) 2873-2920; (c) G.R. Humphrey, J.T. Kuethe, Chem. Rev. 106 (2006) 2875-2911; (d) B.J. Stokes, H. Dong, B.E. Leslie, A.L. Pumphrey, T.G. Driver, J. Am. Chem. Soc. 129 (2007) 7500-7501; (e) M. Ganesh, D. Seidel, J. Am. Chem. Soc. 130 (2008) 16464-16465; (f) M. Bandini, A. Eichholzer, Angew. Chem. Int. Ed. 48 (2009) 9608-9644; (g) C. Guo, J. Song, S. Luo, L. Gong, Angew. Chem. Int. Ed. 49 (2010) 5558-5564; (h) T.P. Pathak, K.M. Gligorich, B.E. Welm, M.S. Sigman, J. Am. Chem. Soc. 132 (2010) 7870-7871; (i) J.B. Chen, Y.X. Jia, Org. Biomol. Chem. 15 (2017) 3550-3567; (j) S. Patil, R. Patil, Curr. Org. Synth. 4 (2007) 201-222; (k) Y.Y. Liu, X.Y. Yu, J.R. Chen, et al., Angew. Chem. Int. Ed. 56 (2017) 9527-9531. |
| [3] |
(a) C. Schultz, A. Link, M. Leost, et al., J. Med. Chem. 42 (1999) 2909-2919; (b) B.W. Trotter, A.G. Quigley, W.C. Lumma, et al., Bioorg. Med. Chem. Lett. 11 (2001) 865-869; (c) D.E. Williams, J. Davies, B.O. Patrick, et al., Org. Lett. 10 (2008) 3501-3504; (d) F.R. de S. Alves, E.J. Barreiro, C.A.M. Fraga, Mini-Rev. Med. Chem. 9 (2009) 782-793. |
| [4] |
(a) R. Gibe, M.A. Kerr, J. Org. Chem. 67 (2002) 6247-6249; (b) Y.J. Lian, H.M.L. Davies, J. Am. Chem. Soc. 132 (2010) 440-441; (c) A. De Angelis, V.W. Shurtleff, O. Dmitrenko, J.M. Fox, J. Am. Chem. Soc. 133 (2011) 1650-1653; (d) Y. Lian, H.M.L. Davies, Org. Lett. 14 (2012) 1934-1937; (e) F. Ye, Z.X. Wang, J. Wang, et al., J. Am. Chem. Soc. 137 (2015) 4435-4444; (f) D.T. Boruta, O. Dmitrenko, J.M. Fox, et al., Chem. Sci. 3 (2012) 1589-1593; (g) H.M.L. Davies, S.J. Hedley, Chem. Soc. Rev. 36 (2007) 1109-1119; (h) D.R. Stuart, P. Alsabeh, M. Kuhnand, K. Fagnou, J. Am. Chem. Soc. 132 (2010) 18326-18339. |
| [5] |
(a) Z. Yu, B. Ma, M. Chen, et al., J. Am. Chem. Soc. 136 (2014) 6904-6907; (b) Y. Xi, Y. Su, Z. Yu, et al., Angew. Chem. Int. Ed. 53 (2014) 9817-9821; (c) R.R. Singh, R.S. Liu, Chem. Commun. 53 (2017) 4593-4596. |
| [6] |
(a) X. Gao, B. Wu, W.X. Huang, M.W. Chen, Y.G. Zhou, Angew. Chem. Int. Ed. 54 (2015) 11956-11960; (b) S. Cacchi, G. Fabrizi, Chem. Rev. 105 (2005) 2873-2920; (c) Z. Hu, S. Luo, Q. Zhu, Adv. Synth. Catal. 357 (2015) 1060-1064; (d) S. Wurtz, S. Rakshit, F. Glorius, et al., Angew. Chem. Int. Ed. 47 (2008) 7230-7233; (e) Z. Shi, C. Zhang, N. Jiao, et al., Angew. Chem. Int. Ed. 48 (2009) 4572-4576. |
| [7] |
(a) M. Delgado-Rebollo, A. Prieto, P.J. Perez, ChemCatChem 6 (2014) 2047-2052; (b) X. Gao, B. Wu, Z. Yan, Y.G. Zhou, Org. Biomol. Chem. 14 (2016) 8237-8240; (c) M.B. Johansen, M.A. Kerr, Org. Lett. 12 (2010) 4956-4959; (d) A. Mark, A.A. Jaworski, K.A. Scheidt, et al., Angew. Chem. Int. Ed. 57 (2018) 17225-17229; (e) Y.Z. Cheng, Q.R. Zhao, X. Zhang, et al., Angew. Chem. Int. Ed. 58 (2019) 18069-18074; (f) J.M. Fraile, K.L. Jeune, J.A. Mayoral, et al., Org. Biomol. Chem. 11 (2013) 4327-4332. |
| [8] |
Y. Cai, S.F. Zhu, G.P. Wang, Q.L. Zhou, Adv.Synth.Catal. 353 (2011) 2939-2944. DOI:10.1002/adsc.201100334 |
| [9] |
D.A. Vargas, A. Tinoco, V. Tyagi, R. Fasan, Angew.Chem.Int.Ed. 57 (2018) 9911-9915. DOI:10.1002/anie.201804779 |
| [10] |
(a) S. Nakagawa, K.A. Bainbridge, K. Butcher, et al., ChemMedChem 7 (2012) 233-236; (b) L. Kupracz, A. Kirschning, J. Flow Chem. 3 (2013) 11-16; (c) D.M. Allwood, D.C. Blakemore, A.D. Brown, S.V. Ley, J. Org. Chem. 79 (2014) 328-338; (d) C.L. Sun, Z.J. Shi, Chem. Rev. 114 (2014) 9219-9280; (e) W. Liu, C.J. Li, Synlett 28 (2017) 2714-2754; (f) W. Liu, J. Li, P. Querard, C.J. Li, J. Am. Chem. Soc. 141 (2019) 6755-6764; (g) J. Chen, D. Chang, F. Xiao, G.J. Deng, J. Org. Chem. 84 (2019) 568-578. |
| [11] |
J. Barluenga, M. Tomas-Gamasa, F. Aznar, C. Valde s, Nat.Chem. 1 (2009) 494-499. DOI:10.1038/nchem.328 |
| [12] |
C. Battilocchio, F. Feist, A. Hafner, S.V. Ley, et al., Nat.Chem. 8 (2016) 360-367. DOI:10.1038/nchem.2439 |
| [13] |
J. Lv, B. Zhao, L. Liu, et al., Adv.Synth.Catal. 360 (2018) 4054-4059. DOI:10.1002/adsc.201800509 |
| [14] |
(a) J. Lv, X. Chen, K.N. Houk, Z. Shi, et al., Nature 575 (2019) 336-340; (b) J. Lv, B. Zhao, Y. Yuan, Y. Han, Z. Shi, Nat. Commun. 11 (2020) 1316. |
| [15] |
A.J. Warner, A. Churn, J.S. McGough, M.J. Ingleson, Angew.Chem.Int.Ed. 56 (2017) 354-358. DOI:10.1002/anie.201610014 |
 2021, Vol. 32
2021, Vol. 32 

