Biological channels in cell membrane control signal transduction and transmembrane transport. For example, when senses the stimulus of the action potential, sodium-potassium pump (Na+-K+ pump) selectively transports 3Na+ ions out of cell membrane, and simultaneously 2K+ ions in, to maintain the resting potential [1]. Similarly, investigators attempt to fabricate artificial nanochannels with the abilities of stimuli-responsive sensing and selective transmembrane transport, as bionic nanochannels.
Different from the fragile biological channels in the cell membrane, many bionic nanochannels are constructed in solid-state membranes for stability [2]. And in order to achieve selective sense or transport, the artificial receptor (as "host") is decorated into bionic nanochannels, which endows them with the selectivity for biological analyte (as "guest") [3]. The host in bionic nanochannels recognizes specific guest or stimulus, then the covalent react or noncovalent binding between host and guest results into the property change of nanochannel, such as the chemical composition, surface charge, wettability and diameter, finally, the bionic nanochannel outputs electrochemical sensing signal [4]. Furthermore, some bionic nanochannels bind with guest transitorily, and the changed property forms a potential gradient in nanochannels [5]. Under the condition, the guest is selectively recognized and bound firstly, then dissociates and transports along the potential gradient in nanochannels [6-8]. Briefly, similar to the signal transduction and transmembrane transport in biological channels, the sensing and transport in bionic nanochannels rely on the host-guest binding. And in many cases of bionic nanochannels, the sensing is to gate the electrical signal, the transport is to drive the mass delivery, and the latter is usually based on the former.
However, the selectivity of ions and chiral molecules remains a challenging problem due to the small size and similar stereostructure [9]. This problem limits the development of sensing and transport in bionic nanochannels. To solve the problem of selectivity, investigators imitate the biological receptor as multilocus amino acid residues and attempt to introduce multisite hosts into bionic nanochannels. Because of the additivity of noncovalent binding increments, the host with multisite for recognition shows better selectivity and sensitivity [10]. The macrocyclic host is one kind of excellent multisite hosts, consisting of crown ethers, cucurbituril, cyclodextrins, calixarenes, pillararenes, etc. [11, 12]. The cavity and easy derivatization enable he macrocyclic host the capability of guest-recognition and stimuli-responsivity. Therefore one kind of bionic nanochannels is constructed as macrocyclic host-modified solid-state nanochannel. And based on the cavity of macrocyclic host and the affinity of the macrocyclic host to the biomaterial, some bionic nanochannels are constructed by embedding macrocyclic host in a phospholipid membrane. Combining with the confinement effect of nanochannel, the macrocyclic host-based nanochannel exhibits more specific and sensitive sensing signal as transmembrane current. And numerous studies have indicated that the macrocyclic host-based nanochannel sensors show extraordinary selectivity to ions and chiral molecules [13-15]. Additionally, based on the dynamic reversible host-guest interaction, the macrocyclic host in nanochannel controls the concentration distribution and gates the transport of ions and chiral molecules. Therefore, the macrocyclic host-based nanochannel is capable of selective sensing and transport for ions and chiral molecules.
Herein, we provide a comprehensive overview of macrocyclic host-based nanochannel on the selective sensing and transport of ions and chiral molecules (Fig. 1). The first section reviews the sensing by macrocyclic host-based nanochannel, aiming at ions and chiral molecules. In the part of the sensing of ions, the property of ionic guests of different macrocyclic hosts is elaborated. In the part of the sensing of chiral molecules, the common guest as amino acids is firstly summarized and following with different kinds of chiral guests of different macrocyclic hosts. The second section reviews the selective transport in the macrocyclic host-based nanochannel, in the sequence of ions and chiral molecules. In conclusion, the comparative evaluation of the study on macrocyclic host-based nanochannels presented here, the potential applications, and development directions in the future have prospected.

|
Download:
|
| Fig. 1. Macrocyclic host-based nanochannel for selective sensing and transport of ions and chiral molecules. | |
As the molecular basis of sensing in nanochannels, macrocyclic hosts selectively and sensitively recognize specific ions and chiral molecules. Crown ethers with different cavity sizes can selectively include different alkali metal ions and form unique 1:1 or 2:1 complexes [16]. Crown ethers are also capable of complexing with ammonium and primary amines by three N—H⋯O hydrogen bonds in a tripod arrangement [17]. Moreover, cyclodextrins (CDs) are capable of selectively recognizing chiral enantiomers and reversibly forminghost-guest inclusion complexes [18]. And the controlled release can also be triggered by external stimuli such as pH, temperature, magnetic field, voltage, as well as molecular competition [19]. Similarly, calixarenes and pillararenes have the same ability of recognizing specific ions and chiral molecules and forming reversible host-guest inclusion complexes with them.
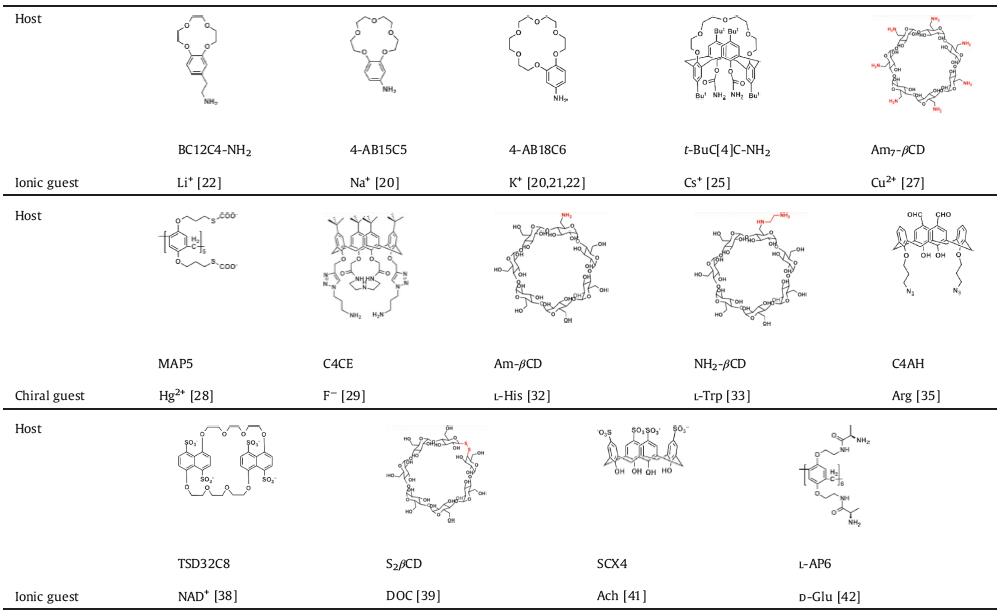
2.1. Ionic sensing by macrocyclic host-based nanochannelTwo biomimetic ionic sensors of Na+ and K+ based on crown ether modified conical nanochannels were reported by Jiang and co-workers [20-22]. The bionic nanochannels recognize Na+ and K+ by the 4′-aminobenzo-15-crown-5 (4-AB15C5) and 4′-aminobenzo-18-crown-6 (4-AB18C6), respectively. The selective host-guest sensing is proved over other alkali metal ions due to the binding of cation size and the crown ether cavity. And the host-guest binding leads to significant changes in the surface charge and wettability, and finally electronic conduction of the nanochannels (Fig. 2). Moreover, the differences in binding strength between these two complexes (the association constant of 4-AB15C5 ⊃ Na+ is 13.2 L/mol as half of 4-AB18C6 ⊃ K+) results in different sensing signals. Na+ regulates the states of ion conduction through the hydrophobic 4-AB15C5 modified nanochannel from close to open, expressed as the increased sensing current. While stronger binding of K+ in 4-AB18C6 reverses the anion conduction to cation conduction, expressed as the reversed rectification of sensing current. In Martin and co-workers' study, the 18-crown-6 modified nanochannel works as a sensor and pump for Pd2+ [23]. Different with the binding of K+ and 4-AB18C6 in Jiang's study, two 18-crown-6 hosts bind one Pd2+ as a sandwich. Even more interestingly, this 18-crown-6 modified nanochannel regulate the nanofluidas a pump, yielding high flow rates at low Pb2+ concentrations (< 1 μmol/L), but decreasing flow rate with concentrations above this threshold.
|
Download:
|
| Fig. 2. Schematic demonstration of the Na+ and K+ regulated ionic nanochannels. I-V characteristics of these two nanochannels before and after activation with Na+ and K+, respectively. Copied with permission [20]. Copyright 2015, American Chemical Society. | |
Based on the same principle of size matching, the selective host of lithium ion (Li+), as the smallest alkali metal ion, is 12-crown-4 with the smallest cavity. Therefore, Ali and Ensinger synthesized aminoethyl-benzo-12-crown-4 (BC12C4-NH2) as the selective host of Li+ and fabricated the BC12C4-NH2 modified nanochannels exhibiting selective sensing of Li+ in the presence of other alkali cations [24]. For cesium ion (Cs+), the macrocyclic host with a larger cavity is required. Therefore, the amine-terminated p-tert-butylcalix[4]arene-crown (t-BuC [4]C-NH2) was synthesized as the selective host of Cs+ and modified into the asymmetric nanochannels [25]. The interior surface of the nanochannel was switched from a hydrophobic and uncharged non-conductive state to a hydrophilic and positive charged conductive state upon the complexation of Cs+. And the conductance of nanochannel increases directly proportional to the concentration of Cs+, suggesting the quantitative ability of this nanochannel sensor.
Similar to the host-guest binding of crown ethers with alkali metal ions, the interaction of CDs with IIA/IIB group metal cations is elaborated by a computational study [26]. The host CD preferentially binds smaller cations with enhanced charge-accepting ability. Kang developed the heptakis-(6-deoxy-6-amino)-β-cyclodextrin (am7-βCD) embedded α-hemolysin (α-HL) channel and achieved the detection of Cu2+ based on the high affinity between Cu2+ and the amino groups of am7-βCD [27]. Different from above crown ether modified solid-state nanochannels, the single molecule of am7-βCD 7 was embedded into the single channel of α-HL. Through the process of fabrication is more sophisticated and complex, the single channel is capable of sensitive sensing of a single ion, expressed as individual pulse current signal when every Cu2+ ion binding with am7-βCD. Further, compared with the macrocyclic host modified solid-state nanochannels, the am7-βCD embedded single nanochannel possesses a low detection limit of 12 nmol/L.
Furthermore, based on the reversible host-guest binding of macrocyclic host and ionic guest, a mercaptoacetic acid-pillar[5]arene (MAP5) self-assembled nanochannels works as the sensor of mercury(II) (Hg2+) as well as an ionic gating [28]. The MAP5 self-assembled nanochannel is highly effective at distinguishing Hg2+ from other divalent metal ions. By virtue of the unique design of the reversible host-guest binding, the ionic conductance in nanochannel is reversibly switched between "on" and "off" in the absence and presence of Hg2+. Therefore, this MAP5 self-assembled nanochannel is capable to detect Hg2+ as a sensor and act as a gate valve for the development of nanoelectronic logic devices.
By regulating the cavity size and the functional groups, calixarenes and pillararenes are also capable of biding with specific anions. For selective sensing of F−, an artificial single conical nanochannel modified with 1, 3-dipropargyl-aza-p-tert-butyl calix[4]crown (C4CE) was designed and established by Li's group [29]. The sensitive recognition from other anions with the detection limit of 9.7 × 10−7 mol/L was reached due to the N—H⋯F hydrogen-bonding interactions. Davis [30] reported a partial-cone calix[4]arene (paco-H) embedded phospholipid membrane, acting as a sensor of Cl−. Compared with the inactive para-substituted analogue (paco-tBu), the Cl− ion selectively interacts with paco-H and transport through the cavity of paco-H, illustrating the necessity of the conformation of the side chain on the partial cone calixarene for the sensing activity of Cl−. Similar work was reported by Hou and co-workers, the pillar[5]arene with terminal positively charged peptides was embedded into phosphatidylcholine bilayer as a unimolecular transmembrane channel [31]. The insertion of pillar[5]arene would destroy the flux of ions and kill cells, with the effective activity concentration against HepG2 cancer cells is measured to be 5.1 μmol/L.
Above all, a law of ionic guest is shown with the evolution of macrocyclic host. The ionic guests of crown ether are mainly alkali metal ion. With the enlargement of the host cavity, the ionic guest of cyclodextrin includes the metal cations from IIA/IIB group. Based on the conformation of the side chain, the ionic guests of calixarene and pillararene are expanded to IIA/IIB group metal cations and VIIA group halogen anions.
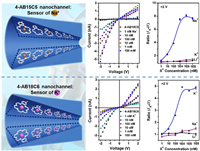
2.2. Chiral molecular sensing by macrocyclic host-based nanochannelThe amino acid is a common chiral guest of macrocyclic hosts. Based on the chemoselectivity and enantioselectivity of CDs for amino acids, Li and co-workers reported a simple enantioselective nanochannel sensor based on the am-βCD covalently modified nanochannel for highly chiral-specific sensing of L-His over D-His and the enantiomers of phenylalanine (Phe) and tyrosine (Tyr) (Fig. 3) [32]. Similarly, Jiang and co-workers reported another β-CD modified nanochannel for sensing of L-Trp [33]. Furthermore, Zhao and co-workers pointed out the considerable L-enantioselectivity of CDs and their derivatives to amino acids, especially to L-histidine (L-His) in aromatic amino acids, L-leucine (L-Leu) in aliphatic amino acids and L-arginine (L-Arg) in alkaline amino acids [34]. Similarly, an aldehyde calix[4]arene (C4AH) modified nanochannel was reported as a sensor of arginine (Arg) over lysine, histidine (His), tryptophan (Trp), glutamine (Gln) and methionine (Met) [35]. Based on the study of the macrocyclic host-based sensing of amino acids, crown ethers and cyclodextrins have been developed as chiral stationary phases for the separation of amino acids [36, 37].
|
Download:
|
| Fig. 3. Schematic demonstration of β-CD-modified single nanochannel for enantioselective sensing of L-His over D-His, L/D-Phe, and L/D-Tyr. Copied with permission [32]. Copyright 2011, American Chemical Society. | |
In addition to common chiral guests as amino acids, macrocyclic hosts possess enantioselective recognition for many different chiral guests. Liu and co-workers synthesized the tetrasulfonated 1, 5-dinaphtho-32-crown-8 with high monovalent affinity and non-preorganized characteristics [38]. This crown ether derivative is able to form complexation with bipyridinium and recognize NAD+ from NADH, which suggests a wider prospect of crown ether in the biomimetic detection of chiral biomolecules.
Furthermore, β-CD and its derivatives are proved as better host molecules of drugs, showing the preference for one of theenantiomers. Such as the investigation reported by Bayley and co-workers, a β-CD disulfide (S2β-CD) lodged α-HL channel could detect sodium deoxycholate (DOC) from a large number of drug guests [39]. Tian and co-workers established a thermoresponsive drug delivery system based on β-CD-modified porous amphiphilic block copolymer films (β-CD-PBCPFs) to selectively load and release doxorubicin (DOX) [40]. These β-CD functionalized devices constructed in different materials suggest a wide application prospect of cyclodextrin-based nanochannel in the controlled release and separation of chiral drugs.
Numerous calixarene/pillararene functionalized nanochannel work as biosensors of amino acids, peptides, and biomolecules. Additionally some of them are improved as a novel method of ionic/molecular transporters and drug delivery systems. Li and co-workers introduced p-sulfonatocalix[4]arene (SCX4) into a single conical nanochannel by layer-by-layer (LBL) assembly, and successfully realized the highly sensitive detection of acetylcholine (Ach) with the detection limit of 1 nmol/L [41].
Then, an L-alanine-decorated pillar[6]arene (L-AP6) self-assembled nanochannel was reported by Li and co-workers [42]. This L-AP6 self-assembled nanochannel work as an efficient chiral-driven ionic gating for glucose enantiomers, it is switched between "off" by D-glucose (D-Glu) and "on" by L-glucose (L-Glu) with good reversibility. Further research indicates that the switching property is due to the differences in binding strength between L-AP6 and glucose enantiomers, where the affinity of self-assembled L-AP6 for D-Glu is 10 times greater than that for L-Glu. And then the more binding of D-Glu with L-AP6 on the interior surface leads to the decreasing of surface charge within nanochannel, therefore the ion transport is restrained (Fig. 4).

|
Download:
|
| Fig. 4. Schematic demonstration of chiral sensing of D-glucoseby L-AP6 nanochannel. Copied with permission [42]. Copyright 2018, Nature. | |
The above reports all suggest the wide prospect of macrocyclic host-based nanochannels in the selective and sensitive sensing, as well as the controlled release and transport of various chiral molecules.
3. Transport of ionic and chiral guests in macrocyclic host-based nanochannelIn the above study of nanochannel sensor, there are some remarkable characters, such as the tubular macrocyclic molecule as a unimolecular transmembrane channel and the ionic gating switched by the reversible binding. These characters suggest the development of macrocyclic host-based nanochannels from sensors to transporters. In addition to the host-guest binding, a dynamic process under the driving force is asked to achieve in the macrocyclic host-based nanochannel for the transport of ions and chiral molecules.
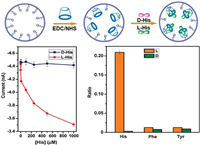
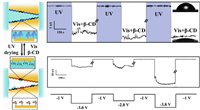
3.1. Ionic transport in macrocyclic host-based nanochannelThe controlled transport of H2O is realized in wettability tunable β-CD self-assembled nanochannels by Jiang and Wen [43]. The hydrophilic β-CD was reversibly self-assembled with the hydrophobic azobenzene (Azo). This host-guest system is usually used as a reversibly photosensitive adjuster, because the trans-Azo under UV light spontaneously assembles with β-CD, while the cis-Azo in visible light is released out. Controlled by light and electric field, the hydrophilic β-CD self-assembled nanochannels switched to the hydrophobic nanochannels with cis-Azo on the interior surface, thus the transport of water was interdicted (Fig. 5). With this addressable way, the surface wetting behavior could be switched between non-conducting and conducting states, which may find applications in optical and electronic logic gating, as well as the controlled mass transport at nano-confined environments.
|
Download:
|
| Fig. 5. Schematic demonstration of reversible optical controlled wettability in nano-confined environments. Copied with permission [43]. Copyright 2018, American Chemical Society. | |
In living systems, ion channels control the species and direction of ionic transmission. Inspired by channel rhodopsins, a light-activated nanochannel switching the ionic transport between cation-selective and anion-selective was established by Li and co-workers [44]. The ionic transport switch was based on the reversible light-controlled self-assembly of a negatively charged pillar[6]arene host (P6A) and a positively charged Azo guest. Later, a facile temperature-controlled self-assembly of carboxylic pillar[5]arene (P5A) and ionic liquid (IL) was introduced into a nanochannel to construct a temperature-sensitive ionic transporter by Li and co-workers [45].
Similarly, ionic transport was switched from cation-selective to anion-selective via the dissociation of the negatively charged host by raising the temperature to 55 ℃ (Fig. 6). During the process of self-assembly and dissociation, the charge polarity in interior surface and the wettability of the nanochannel change, thus the selectivity of ionic transport switches between anion and cation, with the change of ionic conductivity. The above study on the cation-anion-switched transport in polarity tunable pillararene-based nanochannel provides a better strategy for controlled mass transport and separation.

|
Download:
|
| Fig. 6. Schematic demonstration of temperature-sensitive artificial P5A-modified nanochannel, and sensitive transport current of cation and anion. Copied with permission [45]. Copyright 2017, Wiley. | |
Based on the controlled transport of ions in macrocyclic host-based nanochannels, a calixarene-based nanochannel was built by Liu and Zhang to separate multiple heavy-metal ions in water [46]. Because the thiacalix[4]arene-p-tetrasulfonate (TCAS) could selectively form complex with heavy-metal ions according to the prioritization of the selecting order as Cu2+ > Cd2+ > Pb2+ > Ba2+, the TCAS-based nanochannel could separate heavy-metal ions step by step (Fig. 7). During the separation process, the separation speed was proportional to the driven voltage within the threshold, and the pH value influenced the ionic hydrolysis, the complexation of ions to TCAS, and the speed of electroosmotic flow. As a result, at the driven voltage of 1.5 V and the pH value of 5.0, the maximum separation efficiency was achieved as 94.8%, 95.2%, 92.8%, 93.6%, for Cu2+, Cd2+, Pb2+ and Ba2+, respectively. As we know, special selectivity, high efficiency, and low costs are quite important for removing heavymetal ions from wastewater. Therefore the TCAS-based nanochannel provided a controllable and efficient method to membrane separation of heavymetal ions.

|
Download:
|
| Fig. 7. Schematic demonstration of TCAS-based nanochannel membrane for sequential separation of heavymetal ions. Copied with permission [46]. Copyright 2018, American Chemical Society. | |
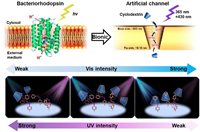
Jiang and co-workers [47] have described an artificial molecule motors system that uses light energy to perform transmembrane molecule transport of CD through Azo modified nanochannels (Fig. 8). The hydrophobicity, reversible photoreaction, and photo-powered rotation-inversion movement of Azo allow the nanochannel to function as a transporter to selectively move the CD molecules across the nanochannel by continuous entrap and release processes. By functionalizing these CD derivatives with other molecular cargo containers, specific host molecules can be selectively transported across the membrane, which will find potential applications in drug delivery and separation. Furthermore, such an artificial light-powered molecular transporter may facilitate a better understanding of the biological machinery.
|
Download:
|
| Fig. 8. Schematic demonstration of bacteriorhodopsin-inspired light-driven artificial molecule motors for transmembrane mass transporting. Copied with permission [47]. Copyright 2018, Weliy. | |
Guo and Hennig [48] reported a phosphorylation-responsive peptide sensor and transporter. The anionic amphiphilic calixarene embedded in the phospholipid membrane can recognize dephosphorylated cell-penetrating peptides (CPPs) over phosphorylated CPPs and process the transmembrane transport. Li and co-workers [49] presented a robust strategy to construct a pH-controlled pillararene self-assembled nanochannels as a specific transporter of histone. Benefit from the design of the N-acetyl-L-cysteine-pillar[5]arene as an artificial receptor, the functional nanochannel well-facilitated histone transport. The histone transport process was switched between "on" and "off" by manipulating the self-assembly and dissociation of the artificial receptor via regulating pH value, which was recorded by patch-clamp technique as the distinct current blocking events.
Drug transport systems based on stimuli-responsive nanochannel membranes, metal-organic frameworks (MOFs), and silica nanoparticles have been extensively investigated. They have advantages such as selectivity of drugs, stimuli-respondence, and controlled release. Tian and co-workers harnessed the thermoresponsive host-guest interactions of β-CD and doxorubicin (DOX) to the nanochannel membrane [40]. The β-CD nanochannel membrane released loaded DOX faster at 37 ℃ than at 25 ℃, while the release was tuned by increasing the β-CD content. Based on different nanochannel material, Yang and co-workers reported pillararene self-assembled metal-organic frameworks (MOFs) [50]. Imitating the biological condition in tumor cells, the lowered pH, increased Ca2+ concentration, and heating up were designed as the stimulus of drug release. Based on the multi-stimuli responsive self-assembly and dissociation of pillararene, the pillararene self-assembled MOFs released the loaded drug 5-fluorouracil under any of these three stimuli. With a similar strategy, they also fabricated the pillararene self-assembled silica nanoparticles that could be activated by different external stimuli. The release of cargo drugs was achieved by lowering the pH or adding a competitive binding agent of the pillararene [51].
Based on the reversible host-guest binding, controlled sensing and transport, macrocyclic host-based nanochannels are improved as smart devices. For instance, utilizing the multi-responsiveness of the CD-Azo self-assembly system to pH, light and temperature, Xia and co-workers fabricate a "plug and play" nanochannel as a smart nanosensor [52]. Jiang and co-workers fabricate an adjustable multiple gating based on the layer-by-layer self-assembly of cucurbit[8]uril [53]. Therefore, based on the extraordinary selectivity, multi-responsiveness and multi-signal output, controllability and reversibility, the macrocyclic host-based nanochannels open up the possibility of developing smart materials for diverse fields, such as seawater desalination, chiral drug separation and new battery material.
4. Conclusions and outlooksThis review summarized the main advances of selective sensing and transport of ions and chiral molecules by macrocyclic host-based nanochannels, and reviewing in terms of the functions and guests. When function as the ionic sensors, the detected ionic guest depends on the cavity size and the functional group of macrocyclic host modified in the nanochannel. The ionic guests of crown ether are mainly alkali metal ion. With the enlargement of the host cavity, the ionic guest of cyclodextrin includes the metal cations from IIA/IIB group. Based on the conformation of the side chain, the ionic guests of calixarene and pillararene are expanded to IIA/IIB group metal cations and VIIA group halogen anions. When function as the chiral molecular sensors, macrocyclic host-based nanochannels possess enantioselective recognition to many different chiral guests, where a common chiral guest is amino acid (Table 1). Based on the reversible host-guest binding researched during the nanochannel sensing, the dynamic transport with external control and drive was investigated in macrocyclic host-based nanochannels. The wettability, charge polarity, conductivity, and the functional host of nanochannels are controlled to regulate the state of mass transport, including the selectivity, direction, and transport rate. Thereinto, the potential of controlled release and separation is revealed during the study on the ionic and chiral molecular transport in macrocyclic host-based nanochannels.
|
|
Table 1 The host-guest systems of macrocyclic hosts and ionic/chiral guests in bionic nanochannel sensing. |
Compared with other functional nanochannels, the macrocyclic host-based nanochannels exhibit extraordinary selectivity to ions and chiral molecules, which is contributed to the size matched cavity and easily derived functional group of the macrocyclic host. Except for the ionic and chiral guests, the macrocyclic host-based nanochannels also respond to photic and thermal stimuli, reflecting the multi-responsiveness and controllability. In addition, the controlled wettability, charge polarity, conductivity and/or surface chemical composition enable the macrocyclic host-based nanochannels as multi-signal output sensors. On the other hand, the reversible host-guest binding enables the sustainable utilization of macrocyclic host-based nanochannels for selective sensing and transport.
Remarkable results of selective sensing and transport for ions and chiral molecules suggest wide potential applications of macrocyclic host-based nanochannels, such as seawater desalination and chiral drug separation. The macrocyclic host self-assembled nanochannels could be designed to selectively bind one configuration from racemic chiral drugs and control it dissociate from the macrocyclic host, finally separate racemic drugs step by step. Therefore, we expect the possible development direction of macrocyclic host-based nanochannels as the smart biomaterials for chiral drug separation in the future. However, there remains room to be improved before the application, such as the selectivity and flux of transport or further separation, and the stability of bionic nanochannel material in practical application. On the other hand, based on the controlled ionic conduction, the macrocyclic host-based nanochannels could be improved as energy materials. For instance, the new battery based on macrocyclic host-based nanochannels membrane could be applied into the transparent screen mobile phone.
In conclusion, the macrocyclic host-based nanochannel, inspired by biological channels in the cell membrane, not only accomplish selective sensing and transport of ions and chiral molecules, but also has bright application prospect on purification and separation. As the scientific community continues to focus on fabricating the bionic nanochannel material with high selectivity, high flux, and high stability, the application of it will surely be realized in the future.
Declaration of competing interestWe declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work.There is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in the review of the manuscript entitled.
AcknowledgmentsThis work was financially supported by the National Key Research and Development Program of China (No. 2018YFD0200102), the National Natural Science Foundation of China (Nos. 21911530178 and 21772055), the 111 Project (No. B17019) and Self-determined research funds of CCNU from the colleges' basic research and operation of MOE.
| [1] |
J.P. Castillo, H. Rui, D. Basilio, et al., Nat. Commun. 6 (2015) 7622-7629. DOI:10.1038/ncomms8622 |
| [2] |
Z. Long, S.S. Zhan, P.C. Gao, et al., Anal. Chem. 90 (2018) 577-588. DOI:10.1021/acs.analchem.7b04737 |
| [3] |
G. Pérez-Mitta, M.E. Toimil-Molares, C. Trautmann, W.A. Marmisollé, O. Azzaroni, Adv. Mater. 31 (2019) 1901483-1901528. DOI:10.1002/adma.201901483 |
| [4] |
G. Perez-Mitta, A.G. Albesa, C. Trautmann, M.E. Toimil-Molares, O. Azzaroni, Chem. Sci. 8 (2017) 890-913. DOI:10.1039/C6SC04255D |
| [5] |
F. Li, T. Ito, J. Am. Chem. Soc. 135 (2013) 16260-16263. DOI:10.1021/ja407002d |
| [6] |
S. Howorka, Z. Siwy, Chem. Soc. Rev. 38 (2009) 2360-2384. DOI:10.1039/b813796j |
| [7] |
L.T. Sexton, L.P. Horne, C.R. Martin, Mol. Syst. Biol. 3 (2007) 667-685. |
| [8] |
X. Shi, R. Gao, Y.L. Ying, et al., ACS Sens. 1 (2016) 1086-1090. DOI:10.1021/acssensors.6b00408 |
| [9] |
J.J. Gooding, K. Gaus, Angew. Chem. Int. Ed. 55 (2016) 11354-11366. DOI:10.1002/anie.201600495 |
| [10] |
W. Zhang, Y.M. Zhang, S.H. Li, et al., Angew. Chem. Int. Ed. 55 (2016) 1-6. DOI:10.1002/anie.201510990 |
| [11] |
Z.C. Liu, S.K.M. Nalluri, J.F. Stoddart, Chem. Soc. Rev. 46 (2017) 2459-2478. DOI:10.1039/C7CS00185A |
| [12] |
H.T.Z. Zhu, L.Q. Shangguan, B.B. Shi, G.C. Yu, F.H. Huang, Mater. Chem. Front. 2 (2018) 2152-2174. DOI:10.1039/C8QM00314A |
| [13] |
Y.F. Wu, D.Y. Wang, I. Willner, Y. Tian, L. Jiang, Angew. Chem. Int. Ed. 57 (2018) 7790-7794. DOI:10.1002/anie.201803222 |
| [14] |
Y.L. Ying, J.J. Zhang, R. Gao, Y.T. Long, Angew. Chem. Int. Ed. 52 (2013) 13154-13161. DOI:10.1002/anie.201303529 |
| [15] |
R. Pinalli, A. Pedrini, E. Dalcanale, Chem. Soc. Rev. 47 (2018) 7006-7026. DOI:10.1039/C8CS00271A |
| [16] |
F.C. Pigge, M.K. Dighe, J.C.D. Houtman, J. Org. Chem. 73 (2008) 2760-2767. DOI:10.1021/jo7026867 |
| [17] |
M. Sarma, T. Chatterje, S.K. Das, RSC Adv. 2 (2012) 3920-3926. DOI:10.1039/c2ra20109g |
| [18] |
K.B. Lipkowitz, R. Coner, M.A. Peterson, A. Morreale, J. Shackelford, J. Org. Chem. 63 (1998) 732-745. DOI:10.1021/jo9717090 |
| [19] |
L. Szente, J. Szeman, Anal. Chem. 85 (2013) 8024-8030. DOI:10.1021/ac400639y |
| [20] |
Q. Liu, K. Xiao, L.P. Wen, et al., J. Am. Chem. Soc. 137 (2015) 11976-11983. DOI:10.1021/jacs.5b04911 |
| [21] |
G. Pérez-Mitta, A.G. Albesa, W. Knoll, et al., Nanoscale 7 (2015) 15594-15598. DOI:10.1039/C5NR04645A |
| [22] |
K. Wu, K. Xiao, L. Chen, et al., Langmuir 33 (2017) 8463-8467. DOI:10.1021/acs.langmuir.7b01705 |
| [23] |
X. Wu, J. Experton, W. Xu, C.R. Martin, Anal. Chem. 90 (2018) 7715-7720. DOI:10.1021/acs.analchem.8b01623 |
| [24] |
M. Ali, I. Ahmed, P. Ramirez, et al., Anal. Chem. 90 (2017) 6820-6826. |
| [25] |
M. Ali, I. Ahmed, P. Ramirez, et al., Langmuir 33 (2017) 9170-9177. DOI:10.1021/acs.langmuir.7b02368 |
| [26] |
S. Angelova, V. Nikolova, N. Moll, T. Dudev, Inorg. Chem. 56 (2017) 1981-1987. DOI:10.1021/acs.inorgchem.6b02564 |
| [27] |
Y.L. Guo, F.F. Jian, X.F. Kang, RSC Adv. 7 (2017) 15315-15320. DOI:10.1039/C7RA00454K |
| [28] |
F. Zhang, J.K. Ma, Y. Sun, et al., Chem. Sci. 7 (2016) 3227-3233. DOI:10.1039/C5SC04726A |
| [29] |
G.R. Nie, Y. Sun, F. Zhang, et al., Chem. Sci. 6 (2015) 5859-5865. DOI:10.1039/C5SC02191J |
| [30] |
J.L. Seganish, P.V. Santacroce, K.J. Salimian, et al., Angew. Chem. Int. Ed. 45 (2006) 3334-3338. DOI:10.1002/anie.200504489 |
| [31] |
J.Y. Chen, W.W. Haoyang, M. Zhang, et al., Faraday Discuss. 209 (2018) 149-159. DOI:10.1039/C8FD00009C |
| [32] |
C.P. Han, X. Hou, H.C. Zhang, et al., J. Am. Chem. Soc. 133 (2011) 7644-7647. DOI:10.1021/ja2004939 |
| [33] |
G.H. Xie, W. Tian, L.P. Wen, et al., Chem. Commun. 51 (2015) 3135-3138. DOI:10.1039/C4CC09577D |
| [34] |
Y. Liu, C.C. You, H.Y. Zhang, Y.L. Zhao, Eur. J. Org. Chem. (2003) 1415-1422. |
| [35] |
M.M. Song, Z.Y. Sun, C.P. Han, et al., Chem. Eur. J. 20 (2014) 1-8. DOI:10.1002/chem.201390210 |
| [36] |
R. Kuhn, Electrophoresis 20 (1999) 2605-2613. |
| [37] |
W.W. Barnhart, X.Y. Xia, R. Jendena, K.H. Gahm, Chirality 25 (2013) 369-378. DOI:10.1002/chir.22146 |
| [38] |
L. Chen, H.Y. Zhang, Y. Liu, J. Org. Chem. 77 (2012) 9766-9773. DOI:10.1021/jo301911w |
| [39] |
W.W. Li, T.D.W. Claridge, Q.H. Li, et al., J. Am. Chem. Soc. 133 (2011) 1987-2001. DOI:10.1021/ja1100867 |
| [40] |
Y.W. Su, J. Dang, H.T. Zhang, Y.Y. Zhang, W. Tian, Langmuir 33 (2017) 7393-7402. DOI:10.1021/acs.langmuir.7b01502 |
| [41] |
L. Wen, Z.Y. Sun, C.P. Han, et al., Chem. Eur. J. 19 (2013) 7686-7690. DOI:10.1002/chem.201300528 |
| [42] |
Y. Sun, F. Zhang, J.X. Quan, et al., Nat. Commun. 9 (2018) 2617-2623. DOI:10.1038/s41467-018-05103-w |
| [43] |
H. Xie, P. Li, Z.J. Zhao, et al., J. Am. Chem. Soc. 140 (2018) 4552-4559. DOI:10.1021/jacs.7b13136 |
| [44] |
Y. Sun, J.K. Ma, F. Zhang, et al., Nat. Commun. 8 (2017) 260-265. DOI:10.1038/s41467-017-00330-z |
| [45] |
R. Wang, Y. Sun, F. Zhang, et al., Angew. Chem. Int. Ed. 56 (2017) 1-6. DOI:10.1002/anie.201610955 |
| [46] |
L. Liu, K. Zhang, Environ. Sci. Technol. 52 (2018) 5884-5891. DOI:10.1021/acs.est.7b06706 |
| [47] |
G.H. Xie, P. Li, Z.J. Zhao, et al., Angew. Chem. Int. Ed. 57 (2018) 10123-10127. DOI:10.1002/anie.201804299 |
| [48] |
A.B. Bon, Y.C. Pan, W.M. Nau, D.S. Guo, A. Hennig, Angew. Chem. Int. Ed. 56 (2017) 15742-15745. DOI:10.1002/anie.201707979 |
| [49] |
F. Zhang, J.K. Ma, Y. Sun, et al., Anal. Chem. 90 (2018) 8270-8275. DOI:10.1021/acs.analchem.8b01948 |
| [50] |
L.L. Tan, N. Song, S.X. Zhang, et al., J. Mater. Chem. B 4 (2016) 135-140. DOI:10.1039/C5TB01789K |
| [51] |
Y.L. Sun, Y.W. Yang, D.X. Chen, et al., Small 9 (2013) 3224-3229. |
| [52] |
N. Liu, C. Li, T. Zhang, et al., Small 13 (2017) 1600287. DOI:10.1002/smll.201600287 |
| [53] |
R. Fang, H. Zhang, L. Yang, et al., J. Am. Chem. Soc. 138 (2016) 16372-16379. DOI:10.1021/jacs.6b09601 |
 2021, Vol. 32
2021, Vol. 32 


 Siyun Zhang is currently a PhD candidate at the Department of Chemistry at Central China Normal University. Her current scientific interest is focused on the fabrication of supermolecular-functionalized nano-channels, and their application on chiral separation.
;
Siyun Zhang is currently a PhD candidate at the Department of Chemistry at Central China Normal University. Her current scientific interest is focused on the fabrication of supermolecular-functionalized nano-channels, and their application on chiral separation.
; Imene Boussouar earned her PhD degree at the Department of Chemistry at Central China Normal University in 2016. Her research interest focuses on the design and fabrication of supermolecular-functionalized nanochannels.
;
Imene Boussouar earned her PhD degree at the Department of Chemistry at Central China Normal University in 2016. Her research interest focuses on the design and fabrication of supermolecular-functionalized nanochannels.
; Haibing Li received PhD from Department of Chemistry and Molecular Science at Wuhan University. After one-year post doctoral fellowship at CNRS, French in 2003, he backed China to work at Huazhong University of Science and Technology. In 2006, he moved to Central China Normal University in Wuhan as a professor. In 2015, he visited University of Utah for half a year. His research interest focuses on supramolecular chemistry on surfaces. To date, his research records 130 scientific publications and 10 domestic and international patents.
Haibing Li received PhD from Department of Chemistry and Molecular Science at Wuhan University. After one-year post doctoral fellowship at CNRS, French in 2003, he backed China to work at Huazhong University of Science and Technology. In 2006, he moved to Central China Normal University in Wuhan as a professor. In 2015, he visited University of Utah for half a year. His research interest focuses on supramolecular chemistry on surfaces. To date, his research records 130 scientific publications and 10 domestic and international patents.