b Beijing Key Laboratory of Water Resources and Environmental Engineering, China University of Geosciences (Beijing), Beijing 100083, China;
c School of Biotechnology and Health Sciences, Wuyi University, Jiangmen 529020, China
Since penicillin entered human sights in 1928, more antibiotics have been discovered and synthesized. Antibiotics are widely used in the prevention and treatment of human, animal and plant diseases, as well as effective additives to promote the growth of livestock and poultry [1-3]. Antibiotics are natural, synthetic and semi-synthetic compounds that can kill or inhibit the growth of bacteria, archaea, viruses, protozoa, microalgae and fungi. According to the chemical structure or action mechanism of antibiotics, they can be classified into different classes, such as β-lactams, quinolones, tetracyclines, macrolides (Table 1 shows common antibiotics and their properties) [4-6].
|
|
Table 1 Commonly used antibiotics and their general characteristics. |
However, most of the antibiotics taken by humans and animals will be excreted in the form of original drugs or metabolites. Therefore, antibiotics are mainly discharged into the environment through human or livestock excreta, aquaculture industry medicines and production wastewater [7-12]. Previous report indicated that high concentrations of quinolones have been detected in wastewater in Argentina, with a maximum concentration of 22,100 ng/L [13]. According to Zhang's survey of 36 commonly used antibiotics in 2013, the total amount of antibiotics used in China was 92,700 tons, of which 60% were discharged into the environment through human and animal excretion activities [14]. Antibiotics that entered the environment not only directly affected various organisms, but also broken the original ecological balance. Antibiotics can induce a large number of drug-resistant bacteria and ultimately affect human health [15-17]. In 2015, a study on investigating the burden of antibiotics in children, reported that nearly 60% of urine samples from 1000 people in the Yangtze River Delta region of China were detected antibiotics [18, 19]. For human health and ecological safety, it is of critical importance to accurately and conveniently determine the concentration of antibiotics in the fields of medicines, food, clinical research, and the environment.
At present, a variety of techniques for detecting antibiotics have been established, including liquid chromatography-mass spectrometry (LC–MS) [20, 21], fluorescence photometry [22, 23], capillary electrophoresis [24], fluorescent labeling [25], etc. These technologies are highly sensitive and selective technologies. However, they require relatively expensive instruments, complicated operating procedures and long detection times. The advantages of the electrochemical method are low cost, rapid response, high sensitivity, easy operation, and suitable for on-site monitoring.
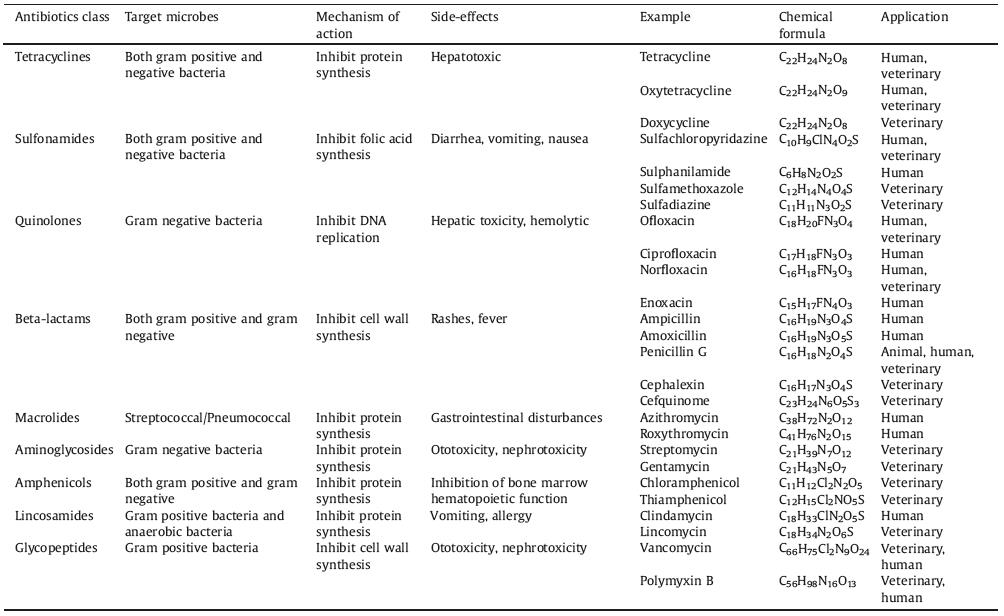
In recent years, there have been numerous reports on the electrochemical detection of antibiotics. We searched the core articles published over the past 20 years by the Web of Science using antibiotics and electrochemical sensors as keywords. Fig. 1 is the trend chart showing the number of core papers on antibiotic/electrochemical sensor published from 2000 to 2019 in Web of Science, and illustrated the application fields of electrochemical sensors at each stage. From Fig. 1, we can find that the trend of electrochemical sensors detecting antibiotics can be divided into three stages. Stage 1 (2000–2009) was the initial stage of electrochemical sensors. The number of published papers at this stage increased slowly and researchers began to mainly explore the electrochemical detection of antibiotics in clinic and medicine. The number of papers published in the stage 2 (2010−2014) increased rapidly. Especially the proportion of articles in food field in all papers has increased significantly. This is because people began to pay more attention to the food safety caused by antibiotics. In the stage 3 (2015–2019), the electrochemical sensor detection of antibiotics was further rapidly developed, and the number of published articles increased greatly. Among them, the proportion of articles on environment field in all papers has increased to 17.04%, which indicates that various environmental pollution caused by antibiotics achieved increasing attention.
|
Download:
|
| Fig. 1. The number of papers on antibiotic & electrochemical sensor published annually in Web of Science core collection (2000-2019) and practical applications of antibiotic electrochemical sensors in fields of (a) environmental monitoring, (b) food safety, (c) clinical diagnoses and (d) microbial resistance detection. | |
Therefore, we need to systematically summarize the development direction and challenges of electrochemical detection of antibiotics. Currently, some review articles on electrochemical detection of antibiotics have been published. Some researchers have reported a review of biosensors for the detection of antibiotics and summarized advances of biosensors based on nanomaterials [26, 27]. However, the preparation methods and modified nano-materials of sensors were mainly described in these reviews. Here, we summarize the literatures on antibiotic detections by various electrochemical sensors in recent years. Firstly, the basic principle of electrochemical sensor for antibiotics and the development of electrode are briefly introduced. We then discuss electrode modification methods, materials and their applications in practical samples. Using the VOSviewer analysis software, we perform statistical and visual network analysis on the literature of antibiotics and electrochemical sensors and analyze the research hotspots. At last, we make a brief summary, with challenges, opportunities and new directions in the future development. We hope that this article can provide not only a comprehensive review in the field of electrochemical detection for antibiotics, but also necessary knowledge, and more importantly, inspires people to further advance this research into practical applications.
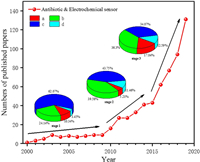
2. The basic principle of electrochemical sensors detecting antibioticsElectrochemical sensor is a device that qualitatively or quantitatively analyzes a target substance. Its essence is the sensing signal generated by the reaction of the measured substance with a specific sensing element, which is converted into an identifiable electrical signal proportional to the concentration of the target substance through a specific transducer. Fig. 2 shows a basic principle of the electrochemical sensor. A complete electrochemical analysis system includes electrochemical sensing equipment, electrochemical detection instrument and electrolyte. Among them, the electrochemical detection instrument, namely the electrode device, usually has a three-electrode structure, including working electrode (WE), reference electrode (RE) and counter electrode (CE) [28].

|
Download:
|
| Fig. 2. Principle of electrochemical sensor (electrode) for detecting antibiotics. | |
Electroanalytical methods for electrochemical detection of antibiotics include voltammetry, electrochemical impedance spectroscopy (EIS), and chronoamperometry (I-t) [29]. There are four voltammetry techniques commonly used in the detection of antibiotics [30, 31]: (i) Cyclic voltammetry (CV), which can see the redox peak potential and current at the same time, and is helpful for studying reversible redox reaction. (ii) Square wave voltammetry (SWV), which applies a fast-scanning stepped voltage on the electrode and is more sensitive than CV. (iii) Differential pulse voltammetry (DPV), which can provide relevant analytes information on the chemical form and the sensitivity also is quite high. (iv) Linear sweep voltammetry (LSV), which is a method of applying a linearly varying voltage to the electrode. EIS is also an effective method for detecting antibiotics, and can also be used to analyze electrode performance [32]. The I-t method is used to frequently detect antibiotics, a test substance undergoes an oxidation-reduction reaction at a constant potential, and records the change of current with time [33].
3. The development of electrochemical sensors for detecting antibioticsGenerally speaking, the working electrode is the core component of an electrochemical sensor, and its performance determines the level of the entire sensor. From this perspective, the development of electrochemical sensors to detect antibiotics has roughly gone through "mercury electrodes, bare solid electrodes, and modified electrodes".
3.1. Mercury electrodeMost of the early electrochemical detection antibiotics used mercury electrodes, including suspended mercury electrodes, mercury drop electrodes, and mercury film electrodes. This is because mercury electrodes are clean, easy to renew, and have good reproducibility [34-36]. In the early stage, Zhou and Pan used a mercury drop electrode or a suspended mercury electrode as working electrodes to quantitatively analyze ofloxacin, and the analysis results were basically consistent with the high-performance liquid chromatography (HPLC) detection results [34]. Mercury electrodes mainly focus on the establishment of detection methods and their application in real samples. Nevertheless, as a volatile and toxic heavy metal, mercury has limited its application in electrochemistry. At present, with very few exceptions, mercury electrodes have basically withdrawn from the field of electrochemical analysis.
3.2. Bare solid electrodeIn order to avoid electrode toxicity, since the 1970s, researchers have begun to use other materials as electrodes. Some metals are not only non-toxic, but also have stable conductive properties, such as gold and platinum. There are also some conductive non-metals, such as glassy carbon electrodes (GCEs), carbon paste electrodes (CPEs) [37-39]. Ambrosi et al. measured ofloxacin and other drugs with carbon paste, gold electrode etc. [37]. In addition to being non-toxic and environmentally friendly, there are many advantages of bare solid electrodes. First, the preparation process is relatively easy, and the surface can be used with a simple pretreatment. Then, due to the wide electrochemical window, carbon electrodes are suitable for a variety of solution systems and enriches the research object. Third, the surface properties of different electrodes lead to differences on the electrochemical behavior of antibiotics, and enrich the related theories of electrode process dynamics. Nevertheless, bare solid electrodes also have obvious shortcomings. The lack of special functional groups on their surfaces results in poor enrichment and catalytic performance. Compared with mercury electrode, its repeatability and stability are poor.
3.3. Modified electrodeIn the past, researchers were mainly concentrated on studying the bare solid electrode. Compared with modified electrodes, the surface properties of bare solid electrodes have certain limitations, such as smaller specific surface area, fewer surface functional groups and poorer specificity [40, 41]. These shortcomings are not conducive to the adsorption and catalytic oxidation of the substance to be tested on the electrode surface. Therefore, researchers have focused more on chemical or chemically modified electrodes [42-45]. It can be expressed as a solid electrode with a specific can be expressed as a solid electrode with a specific microstructure after the surface has been artificially modified and has a significant performance improvement. Wu et al. modified nano-Cu2O, nitrogen-hybrid graphene (GR) and perfluorosulfonic acid (Nafion) on GCE, and the constructed sensor can be used to detect ofloxacin in medicine [46]. Cu2O is a p-type semiconductor with catalytic activity, but has the disadvantage of poor conductivity. While, nitrogen-doped GR has good conductivity and a large surface area. The composite film was found to exhibit a strong electrocatalytic activity towards ofloxacin to yield a detection limit of 0.34 μmol/L. Further, the sensor was also found to exhibit excellent selectivity and standard recoveries (94.0%–104.0%), making its suitable for ofloxacin detection in medicine samples. The excellent sensitivity of gold nanoparticles (AuNPs) has been commonly combined with bio-recognition elements to achieve diverse electrochemical sensing platforms for antibiotic detection. In this regard, Chullasat et al. used AuNPs, antibodies, sulfhydryl succinic acid and thiourea to construct an electrochemical aptamer sensor for highly sensitive and selective detection of chloramphenicol [47]. In addition to the materials mentioned above, there are many other materials used for electrode modification, such as quantum dots, conductive organics. We will discuss in detail in part 5. The synergistic catalysis of multiple materials can improve the electrochemical performance of the electrodes. However, simple modification methods and economical modification materials should be used as much as possible. Up to now, the modified electrodes are the main research direction. Most researchers are using a variety of modified materials and modified methods to prepare the modified electrodes.
4. Preparation of modified electrodeThe surface modification of bare electrode can improve the detection performance. On the one hand, specific trimming and modification of the electrode surface by physical or chemical means can improve the hydrophilicity of the interface between the electrode and the solution and increase the concentration of antibiotics in the reaction area [48-50]. On the other hand, single-layer or multi-layer functional materials, such as nanomaterials, organic materials, enzymes or antibodies, are constructed on the electrode surface through self-assembly, coating, electrodeposition, electropolymerization and other modifications [51-54]. After modification, the electrode will show special surface effect, size effect, quantum tunneling effect and catalytic activity. These specific properties can enhance the electrical conductivity of the electrode, accelerate the transfer of electrons in the interface, reduce the redox overpotential of antibiotics, and promote the electrochemical reaction. The effective combination of the above two aspects can play a synergistic effect and greatly improve the sensitivity of antibiotic detection. According to different reactions occurring insides or surfaces of working electrodes, preparation methods required for modified electrodes can be roughly divided into two main types, top-down and bottom-up.
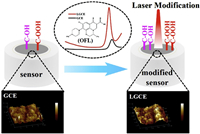
Top-down is a modification used to etch the surface of a solid electrode by laser or other etching methods [48, 49, 55]. By top-down modification, the surface roughness of the electrode can be suitably increased and surface functional groups can be introduced, so as to improve the adsorption performance and electrochemical activity of the electrode. Feng et al. used a laser one-step method to etch directly on GCE, and the modified electrode can detect ofloxacin in real water samples [48]. From Fig. 3, it can be seen that the surface roughness of the electrode increases, that is, the reaction area increases after modification, although the crystal structure does not change. Oxygen-containing functional groups on the surface are increased, which can improve the surface reactivity of the electrode. Chen et al. used the nitrogen plasma technique to bombard the surface of GCEs for the first time, properly increasing the specific surface area and introducing nitrogen reactive functional groups. This modified electrode was effectively applied to the detection of ciprofloxacin in actual water samples [49].
|
Download:
|
| Fig. 3. Schematic illustration of electrochemical sensor for ofloxacin (OFL) detection. GCEs were directly modified using a picosecond laser. This can also improve the electrochemical activity of the electrode interface by increasing the surface roughness and oxygen-containing functional groups. Copied with permission [48]. Copyright 2020, Springer Nature. | |
Bottom-up is modifying other active materials to the electrode surface by self-assembly [56], coating [57, 58], electrochemical polymerization [59, 60] and electrochemical deposition [61], etc., to improve the sensitivity and specificity of electrodes. Self-assembly is a technique in which basic structural units (molecules, nanomaterials, microns or larger) spontaneously form ordered structures. This method is often used to prepare modified electrodes for electrochemical sensors [62]. The coating method is one of the most commonly used modification methods. It is also an adsorption, but it is easier to implement than the self-assembly method. The coating method includes drop coating and dip coating. Drop-coating is application of a quantitative mixture of modified solution and material to the surface of the exposed electrode. To detect oxytetracycline in food, Sun et al. applied self-made Zn-Mt colloidal solution onto the pretreated GCE surface, and then dried it under an infrared lamp to finally obtain a modified electrode [41]. The dip coating method is immersing the electrode in the electrode modification solution, but the dip coating method is not easy to control the amount. Both of these coating methods require electrodes to be dried.
Electrochemical polymerization method and electrochemical deposition method are two very commonly used modification methods. In the electrochemical polymerization method, the electrode is placed in a monomer solution to carry out the polymerization reaction by electrolysis. Wen et al. obtained PoAP/MWCNTs/GCE by electrochemical polymerization of aminophenol and MWCNT mixed solution on the GCE surface at from −0.20V to 0.84V cyclic potential for the determination of levofloxacin in drugs [60]. Electrochemical polymerization is often used in molecularly imprinted sensors. Jafari et al. constructed a molecularly imprinted electrochemical sensor for detecting azithromycin in serum. The sensor was prepared by electropolymerization of aniline on graphene oxide (GO) and gold nanoparticle modified GCE with azithromycin as the template, which can specifically recognize azithromycin [63]. Electrochemical deposition is a technique in which positive and negative ions in electrolyte solution migrate under the action of an external electric field and redox reaction occurs on the electrode to form a coating. Jiang et al. deposited Pt-Au onto the surface of GCE through electrochemical reduction. The actual sample analysis shows that GCE/rGO/Pt-Au can be used to detect ofloxacin in tablets and human urine samples [64]. Electrochemical deposition method is generally used when the electrode is modified by metal or metal oxide nanomaterials.
There are also methods for preparing electrodes apart from the above modification methods, for example, screen-printed electrodes (SPEs), carbon paste electrodes (CPEs), etc. The preparation of SPEs is to deposit ink into a thin film on a solid substrate through a screen board, and different materials can be added to the ink as needed [65]. SPEs can be mass-produced under fixed procedures at low cost, or they can be further modified with other materials after production as needed [66]. Additionally, a common method for preparing CPEs is incorporation. Incorporation is to mix and grind a certain amount of modifier, binder and graphite powder into a glass tube to prepare a chemically modified electrode [67, 68].
5. Modified materialsThe performance of electrochemical sensors for detecting antibiotics depends largely on the performance of working electrodes (WEs), while the performance of WEs depends on the nature of the electrode material. Currently, commonly used modified materials are inorganic nano-materials, bio-functional materials and organic additives. Inorganic nanomaterials include carbon nanomaterials, metal nanomaterials, metal oxide nanomaterials and quantum dots. Table 2 [31, 33, 43, 51, 63, 67, 69-104] shows a series of common modified materials and additives and their application in electrode modification.
|
|
Table 2 Performance analysis of different materials for electrochemical sensors of antibiotics. |
As a class of material to modify electrodes by bottom-up methods, carbon nanomaterials have attracted widespread attention. They have unique characteristics, such as large specific surface area, low background current, wide electrochemical window, high electronic conductivity, good chemical stability, selectivity and reproducibility, so they are suitable for the detection of antibiotic electrochemistry device. Carbon nanomaterials commonly used in the electrochemical detection of antibiotics include carbon nanotubes (CNTs), GR, carbon nanospheres, etc. [105]. Among them, CNTs and GR have been widely used in the detection of antibiotics by electrochemical sensors.
One-dimensional (1D) CNTs have large surface area and high electron transfer rate, which enable electrons to move rapidly from analyte to electrode surface, and have good chemical stability and mechanical strength. Based on these characteristics, CNTs have great potential in electrochemical sensors. According to the number of layers of the tube wall, CNTs are roughly divided into single-wall carbon nanotubes (SWCNTs) and multi-wall carbon nanotubes (MWCNTs). For example, Liu et al. used MWCNTs and other materials to prepare the MWCNTs-CPE/prGO-ANSA/Au composite electrode, which can effectively detect norfloxacin [74]. Chemically cleaving CNTs with an oxidizing agent (strong acid) can graft some groups (carboxyl groups, hydroxyl groups) on the surface of CNTs, which can improve the dispersibility of CNTs. Schebeliski et al. treated the ceramic electrode modified by MWCNTs with acid (HNO3/HClO4) and characterized the modified electrode by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), transmission electron microscopy (SEM) and Raman spectroscopy, and they found that MWCNTs had good dispersion on the ceramic electrode [71].
Since GR was first isolated in 2004, it has become a pioneer in the new materials industry and has been successfully applied in various industries, including sensors. GR is a two-dimensional (2D) nano-thin film with good conductivity and large specific surface area. To detect sulfonamides, He et al. proposed a functionalized graphene and gold nanoparticles modified GCE [77]. Because both GR and gold nanoparticles have large specific surfaces and strong conductivity, sulfonamide molecules tend to accumulate on the electrode surface, indicating that the electrode can enhance the electrochemical response signal. Similar to CNTs, GR is also easy to functionally modify. Embedding functional groups on the base or/and edge of GR can cause structural defects on the GR sheet and give GR new characteristics [106]. For example, GR is treated with an oxidant to obtain GO. Santos et al. used H2SO4/HNO3 to oxidize GR into GO, then they modified GO and nickel oxide nanoparticles (NiO-NPs) on the GCE by electrodeposition. They established a simple, highly selective electrochemical method for the simultaneous determination of acetaminophen (PAR) and ciprofloxacin (CIP) [89]. Wong et al. explored GO and ionic liquids (ILs) modified CPE. The modified CPE increased the analytical sensitivity of ofloxacin by 8.2 times compared to unmodified CPE [79]. However, 2D GR also has some shortcomings. Due to the strong π-π bonds in 2D GR, the graphene layers are very easy to aggregate. The aggregation reduces the effective surface area of the electrode [107]. In addition, the aspect ratio of 2D GR limits the diffusion of ions in its cross section. Compared with 2D GR, three-dimensional (3D) GR has a larger specific surface area and wider space to transfer electrons [108].
In recent years, researchers have developed various technologies for manufacturing 3D GR, which has also been continuously applied in the field of sensors. Zhang et al. assembled GO by spontaneous reduction of zinc foil at room temperature, and then washed it with diluted hydrogen chloride solution to produce 3D rGO. The constructed 3D rGO/GCE can be used for the detection of chloramphenicol [109]. To date, a variety of techniques for preparing 3D GR have been developed, such as laser-induced GR [110]. 3D GR has achieved good results in electrochemical detection of other substances and has a good prospect in the electrochemical detection of antibiotics.
5.2. Metal and metal oxide nanomaterialsMetals have good electrical conductivity, while nano-sized metals have some quantum size effects and quantum tunneling effects caused by smaller particle sizes. Therefore, compared with metal materials, metal nanomaterials have produced some unique changes in physical and chemical properties, such as high specific surface area, rich reaction sites, and good catalytic activity. At the same time, metal nanoparticles can be well combined with other modified materials. Thereby, they can effectively amplify the pulse signals of electrochemical sensors. Noble metal nanoparticles, such as gold, silver and platinum, are widely used to increase the detection limit in the study of electrochemical sensors to detect antibiotics [84, 111, 112]. For example, Roushani et al. explored silver nanoparticles (AgNPs) and 3-aminomethylpyridine functionalized graphene oxide (3-Ampi-GO) as electrochemical detection amplifiers to construct an aptamers and molecularly imprinted dual recognition systems electrochemical sensor for chloramphenicol [113]. In addition to precious metals, transition metals also have a highly efficient catalytic effect. Compared with precious metals, transition metals are more economical and easily available. Gan et al. modified the montmorillonite (MMT) with iron/zinc (Fe/Zn-MMT) by cation exchange and modified it on the surface of GCE to prepare an electrochemical sensor for detecting tetracycline (TCs) [40]. The expanded layer structure of MMT provides a large specific surface area, and Fe/Zn can enhance the catalytic activity. The combination of the two can greatly improve the enrichment ability and electrocatalytic ability of the electrode.
Metal nanomaterials are not stable enough to anti-oxidize in aqueous solutions. However, nano metal oxides have the advantages of chemical inertness, good thermal stability, and low toxicity. With their large specific surface area and good biocompatibility, they can be used as excellent electrode modification materials. Common metal oxide nanomaterials are ZnO, CuO, Fe3O4, etc. ZnO's excellent biocompatibility and non-toxicity have been widely used in nano-sensors, light-emitting diodes and other fields. Sebastian et al. developed a sensor based on GO and ZnO nanomaterials to determine chloramphenicol [114]. The GO/ZnO/GCE sensor has been proven to have high sensitivity and has been successfully used in the actual detection of food and eye drops. Wu et al. prepared CuO/nitrogen graphite nanocomposites by a one-step chemical reduction method, combined with Nafion to modify GCEs, and monitored ofloxacin in real samples [46]. In view of the large surface area, non-toxic, low cost and magnetic properties of Fe3O4, Bhengo et al. combined Fe3O4 and MWCNTs to simultaneously determine sulfamethoxazole and trimethoprim in aqueous solutions [115]. Fe3O4 is considered as an ideal modifier, but it is prone to aggregation and has no selectivity to complex substrates. Therefore, it is necessary to use in combination with other decorative materials.
5.3. Quantum dotsQuantum dots (QDs) are a type of semiconductor nanocrystals whose sizes are less than 10 nm, and also have many advantages (such as high aspect ratio, biocompatibility, catalytic activity, luminescence, etc.) [116]. The role of quantum dots in sensors is mainly achieved through the cooperation of quantum dots and other composite materials. Santos et al. modified GCE with carbon black and CdTe QD in the chitosan membrane. This electrode was successfully used to detect norfloxacin [93]. Roushani et al. reported the use of ZnS QDs and gold nanorod composites to construct a streptomycin aptamer sensor, which has good selectivity for streptomycin [117]. These studies indicate that the combination of quantum dots, carbon materials, metal materials, and bio-functional materials on electrochemical sensors provides a promising direction for detecting low concentrations of antibiotics in different matrices.
5.4. Bio-functional materialsIn the process of preparing electrochemical sensors, except some inorganic chemical materials, biofunctionalized materials are used, such as antigens (antibodies), nucleic acids, and enzymes. They have a high degree of specificity and specifically identify detected substance. In an electrochemical sensor using enzyme biomacromolecule as modified material, the specific reaction can be detected by the catalytic reaction of the enzyme on relevant antibiotics. Que et al. prepared molecular imprinted polymer (MIP) by electropolymerization of aniline and o-phenylenediamine on the gold electrode substrate, and detected streptomycin by signal amplification of glucose oxidase [118]. To a certain extent, enzyme biomacromolecules expand the electrochemical field of antibiotic detection, but the ease of enzyme inactivation still has certain limitations. This type of sensor that reacts specifically with an analyte by immobilizing an antigen (antibody) and then changes in electrical signal is an electrochemical immunosensor. Conzuelo et al. developed an amperometric immune sensor to detect tetracyclines in milk samples using a selective capture of the antibody surface immobilized protein G-functionalized magnetic beads and a screen-printed carbon electrode [119]. The electrochemical aptamer sensor is a biosensor that modifies the nucleic acid aptamer of the corresponding antibiotic on a substrate material with good electrical conductivity. Due to the limited signal gain of traditional aptamers, Yu et al. added a displacement probe and analyzed the effect of displacement probe length and melting temperature on the performance of the sensor. The results showed that using the optimal length of displacement probe is necessary [120]. Modification of metal materials, carbon materials, etc. on the prepared biosensor can further improve the sensitivity of the sensor. Increasing the effective active area of the electrode surface to load more biological macromolecules will help the sensor to be reused for many times over the long term.
5.5. OthersAside from the above materials, some organic materials (such as polyaniline) are often used in the preparation of MIP electrodes. In addition, some conductive organic compounds (such as Nafion reagent, chitosan) are often added during electrode preparation. As a conductive polymer, polyaniline has been widely used in the field of electrochemical sensing. Chen et al. studied combining MoS2 with aniline to prepare materials with good conductivity and large electroactive reaction sites for electrochemical detection of chloramphenicol [121]. However, polyaniline also has shortcomings such as insolubility and high brittleness. In order to solve these problems, aniline derivatives have been studied [118]. Nafion is an electrode modifier called perfluorosulfonated cation exchanger. It can form a film on the electrode surface, whose film has good antifouling ability [122]. Huang et al. constructed MWCNTs/Nafion/GCE to determine norfloxacin, and proved that the modified electrode has fast response speed, repeatability and good stability [123]. Chitosan also has the characteristics of conductivity, biocompatibility and film-forming ability. Moreover, it also contains a large number of amino and hydroxyl functional groups [124]. Liu et al. reported a label-free tetracycline electrochemical immunosensor immobilizing tetracycline monoclonal antibody on the surface of carboxy-Fe3O4MNPs chitosan composite membrane [125].
As the single nature of bare electrode materials limits the performance of electrodes, we usually modify the electrode surfaces to improve their performance. The above-mentioned materials are currently commonly modified materials for antibiotic electrochemical sensors. With the development of science, more new materials will be used in the field of sensing. Different materials have their specific advantages, and it is difficult for a single modified material to achieve the desired goals. Therefore, we can assemble different materials together by some means, which can further enhance the performance of the electrode. For example, metal or metal oxide nanoparticles are relatively easy to agglomerate, and the layered structure of GR can provide many binding sites, which can effectively disperse these nanoparticles. Bio-functional materials have strong specificity, while metal and carbon materials have good catalytic activity and biocompatibility, so they can be used in combination to improve the sensitivity of the electrode. Fig. 4 shows nanocomposites with different structures [61, 83, 99, 126]. It is worth noting that when modifying the electrode, instead of stacking various materials, it is to make full use of the characteristics of the materials. Then, we should also consider the following factors: physical and chemical properties of materials, environmental protection, cost and availability.

|
Download:
|
| Fig. 4. Scanning electron micrographs images of different morphologies of nanocomposite modified materials. (a) Pt/ PoAP/MWCNTs electrodes. Copied with permission [83]. Copyright 2014, Springer Nature. (b) Mixed self-assembled microgels/GO/GCE. Copied with permission [99]. Copyright 2019, American Chemical Society. (c) PAR/EGR. Copied with permission [61]. Copyright 2014, Elsevier. (d) Fe3O4/CF. Copied with permission [126]. Copyright 2019, Springer Nature. | |
The reliability and stability of the actual sample test data is the key to evaluating the performance of an electrochemical sensor. To obtain stable and reliable detection data, we should not only pay attention to electrode preparation, but also sample preparation and method optimization.
6.1. Sample preparationAt present, antibiotic electrochemical sensors have been applied in food, environment, human body fluids and medicine. In order to obtain more accurate testing data, some impurities that may affect the electrode surface should be removed as far as possible. When analyzing real samples, these samples are generally subjected to a simple pretreatment. Analysis environment of the electrochemical sensor is a solution environment, in some cases, solid samples (such as meat, tablets) need to be crushed and made into solutions for analysis.
In the field of food industry, electrochemical sensors are often used to detect antibiotics in meat, honey, milk and other foods. For solid samples (such as chicken), they must be chopped before proceeding to the next step. The chicken sample was processed according to the method of He et al. The 0.5 g chopped chicken was put into the centrifuge tube and 1 mL ethyl acetate was added for ultrasonic treatment for 30 min. Then the mixture was centrifuged at 10,000 rpm for 10 min to collect the supernatant for later use. Although honey and milk are liquid, they also need to be pretreated [111]. The 0.5 g chopped chicken was put into the centrifuge tube and 1 mL ethyl acetate was added for ultrasonic treatment for 30 min. Then the mixture was centrifuged at 10,000 rpm for 10 min to collect the supernatant for later use. The supernatant was then centrifuged at 10,000 rpm for 10 min to collect for later use. Although honey and milk are liquid, they must be pretreated. Huang et al. treated honey samples as follows: 5 g of honey was added with 20 mL of buffer solution containing antibiotics, which was thoroughly mixed with shaking and ultrasonic homogenization. The mixture was then centrifuged (4000 rpm) for 20 min, and the supernatant was collected [127].
Electrochemical sensors can also be used in biology and the level of antibiotics in the human body. Tablets are relatively simple to handle, weighing, grinding, dissolving, and diluting. For serum, 0.15 mL perchloric acid was added to 1 mL blood sample, which was vortex mixed for 1 min and centrifuged at samples were collected in sterile bottles to avoid contamination, centrifuged (3000 rpm, 10 min), and some proteins were removed without further pretreatment [128].
Since most antibiotics are released into the environment, the concentration of antibiotics in the environment is worthy of attention. Antibiotics are primarily released into the water environment. Water samples pretreatment were relatively simple. After water samples were collected according to the standard, they were filtered through 0.45 μmol/L polytetrafluoroethylene membrane to remove physical impurities and keep for reserve.
During pretreatment, antibiotics of different concentrations are added to the samples to evaluate the accuracy of the results. Other instruments can also be used to assist in verifying the results, such as HPLC-MS, capillary electrophoresis.
6.2. Stability assessment and method optimizationSamples pretreatments are used to reduce the interfering substances during sample analysis, but there are still some other interfering factors. Such as heavy metal ions, inorganic salts and other coexisted antibiotics. In order to evaluate the stability of NP-GCE, Chen et al. used four antibiotics and six water ions (sulfapyridine, moxifloxacin, doxycycline, tetracycline, Ca2+, K+, Mg2+, Na+ and Cl−) evaluated the stability of the electrode [49]. Compared with the original peak current, the quinolone antibiotic moxifloxacin has the greatest interference on the detection of ciprofloxacin. The real sample is a complex system. To evaluate the stability of an electrode, some anti-interference experiments need to be done, but it was not done in many studies.
The pH value of electrolyte is an important index, which had great influence on the value of peak current and peak potential. In electrochemical sensors, the pH value mainly affects the activity of electrode materials [129]. Because the form of a substance in an aqueous solution depends on the solution pH, the pH also affects the existence form of antibiotics. Liu et al. firstly combined experimental and simulation methods to explain that ofloxacin is mainly concentrated on the electrode surface through hydrogen bonding and coulomb electrostatic force. Under the combined action of the two forces, the current peak is the largest when the solution pH is 6.0 [130]. In short, the various water environmental parameters in the detection medium have a greater impact on the antibiotic detection by the electrochemical sensor, and should be fully optimized in the detection of real samples.
6.3. Samples analysisIn order to evaluate the performance of electrochemical sensors for detecting antibiotics in practical application, different concentrations of antibiotics are added to the original real samples before pretreatment. It is worth noting that the sample dilution factor during pretreatment needs to be considered. The accuracy of the results is evaluated by the recoveries. Of course, other detection method, such as HPLC, can also be used to verify the accuracy of the results.
In Yari's work, the developed electrochemical sensor for detecting sulfamethoxazole was used on different samples such as pharmaceutical and urine. The serum samples were spiked with standard sulfamethoxazole solution (10, 20, 30, 40 μmol/L). The recoveries were reasonable, 97%–104%. In this work, the standard addition was carried out after the pretreatment. On the other hand, two different co-trimoxazole tablets were analyzed using the standard addition method. An HPLC method was used to measure the sulfamethoxazole contents as a standard method. The results obtained by the electrochemical sensor are compared with the HPLC method. There is no significant difference between the results given by the electrochemical sensor and the HPLC method [73].
He et al. developed an electrochemical sensor for detecting sulfanilamide in pork extract. The spiking was performed after the pretreatment and the samples were diluted with buffer before analysis. The samples were spiked with standard sulfanilamide solution (3.0, 6.0, and 9.0mol/L). The results showed that in three parallel experiments, the average recovery rate was 91.22%–96.67%. Therefore, it showed that the sensor can successfully detect sulfanilamide in real samples [71]. On another work, the tap water samples and sewage plant samples were spiked with standard ofloxacin solution (0.5 μmol/L). The recovery rate of the tap water sample was 96%, the recovery rate of the sewage sample was 93.6% (n = 3) with relative standard division (RSD) value was lower than 6.8%. This shows that the developed aptamer sensor has broad prospects for practical application in actual water samples [86]
Recovery rate and RSD can reflect the accuracy and repeatability of the designed electrochemical sensor. The recovery rate in most of the literatures can reach the standard. However, some literatures lack of the accuracy and repeatability verification. In short, accurate pretreatment and stability evaluation must be carried out before actual sample testing to avoid interference and large errors.
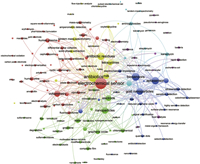
7. Conclusion and prospectNowadays, due to the emergence of various super bacteria and resistance gene, the problem of antibiotic pollution has attracted much attention. Therefore, it is necessary to detect antibiotics in food, clinic and environment. Compared with other antibiotic detection technologies, electrochemical methods are relatively simple and convenient, and also has the most potential for development in situ monitoring. In order to grasp the research hotspots and trends of electrochemical sensors to detect antibiotics, we used the method of bibliometrics to analyze the keywords. The data source for these keywords is the Web of Science core collection. We analyzed the articles on electrochemical detection of antibiotics from 1982 to the present, and analyzed the keywords using VOSviewer analysis software. In the retrieved 1029 papers, keywords with a frequency of more than 10 times were clustered (Fig. 5), mainly including electrochemical sensors, antibiotics, biosensors and nanoparticles. It can be clearly seen that biosensors and nano-material modified electrodes are still the current research hotspots. Tetracyclines, aminoglycosides, macrolides, and quinolone antibiotics have been studied relatively more. This may be because these antibiotics have a higher detection rate in milk, plasma, and water environments. Voltammetry method was often used. The shortcoming of the current research is that it only includes the Web of Science core collection literature. Although some professional terms have been merged, bias may still exist.
|
Download:
|
| Fig. 5. Co-occurrence network map of keywords about research on electrochemical sensors for antibiotics. | |
In this review, we mainly introduced the research progress of electrochemical sensors in the detection of antibiotics and its application in actual sample detection. Because the electrode is the core part of the entire sensor, we summarized the development process of the electrode for antibiotic detection, and described the modification method and materials in detail. In the electrode modification process, we summarized these modification methods into two categories, top-down and bottom-up. We conducted a detailed comparison between various materials, and confirmed that various materials have different properties, and composite materials are the most commonly used and the most effective materials. The real samples pretreatments and detection methods are also explained. Although these electrochemical sensors have some advantages over traditional detection methods, there are still some challenges in the detection of real samples.
A large amount of research is based on the continuous development of modified solid electrodes. These sensors only can need to detect one or two antibiotics, while there are dozens or even dozens of antibiotics in the environment. However, the currently prepared electrodes cannot detect all antibiotics simultaneously, and can only specifically detect one or several kinds of antibiotics. The presence of other antibiotics can interfere with target antibiotic. Moreover, the concentration of antibiotics in the environment is very low, which is much lower than that of antibiotics in food, medicine and other fields. It is generally nmol level, which poses a great challenge to electrode sensitivity. Based on above discussion, we can conclude that electrochemical detection of antibiotics is a cheap and simple method, but further improvement is needed in terms of anti-interference ability and sensitivity. Therefore, future research on electrochemical detection of antibiotics could be based on these aspects.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis study was supported by the Fundamental Research Fund for the Central Universities (Nos. 2652019293, 2652019115), Guangxi Key Research Project (No. GuikeAB18050026), National Natural Science Foundation of China (No. 41731282).
Appendix A.Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.10.025.
| [1] |
K.K. Brandt, A. Amezquita, T. Backhaus, et al., Environ. Int. 85 (2015) 189-205. DOI:10.1016/j.envint.2015.09.013 |
| [2] |
P.S. McManus, V.O. Stockwell, G.W. Sundin, A.L. Jones, Annu. Rev. Phytopathol. 40 (2002) 443-465. DOI:10.1146/annurev.phyto.40.120301.093927 |
| [3] |
F.C. Cabello, Environ. Microbiol. 8 (2006) 1137-1144. DOI:10.1111/j.1462-2920.2006.01054.x |
| [4] |
K. Kummerer, Chemosphere 75 (2009) 417-434. DOI:10.1016/j.chemosphere.2008.11.086 |
| [5] |
M. Kumar, S. Jaiswal, K.K. Sodhi, et al., Environ. Int. 124 (2019) 448-461. DOI:10.1016/j.envint.2018.12.065 |
| [6] |
J.F. Yan, J. Li, J.L. Peng, et al., Chem. Eng. J. 359 (2019) 1097-1110. DOI:10.1016/j.cej.2018.11.074 |
| [7] |
A. Jia, Y. Wan, Y. Xiao, J. Hu, Water Res. 46 (2012) 387-394. DOI:10.1016/j.watres.2011.10.055 |
| [8] |
F. Adachi, A. Yamamoto, K.I. Takakura, R. Kawahara, Sci. Total Environ. 444 (2013) 508-514. DOI:10.1016/j.scitotenv.2012.11.077 |
| [9] |
J. Du, H.X. Zhao, S.S. Liu, et al., Sci. Total Environ. 595 (2017) 521-527. DOI:10.1016/j.scitotenv.2017.03.281 |
| [10] |
J. Cao, Z. Xiong, B. Lai, Chem. Eng. J. 343 (2018) 492-499. DOI:10.1016/j.cej.2018.03.036 |
| [11] |
F.Y. Huang, Z.Y. An, M.J. Moran, F. Liu, J. Hazard. Mater. 399 (2020) 122813. DOI:10.1016/j.jhazmat.2020.122813 |
| [12] |
T.W. Wu, Q. Xue, F. Liu, et al., Chem. Eng. J. 366 (2019) 577-586. DOI:10.1016/j.cej.2019.02.128 |
| [13] |
C.M. Teglia, F.A. Perez, N. Michlig, et al., Environ. Toxicol. Chem. 38 (2019) 2305-2313. DOI:10.1002/etc.4532 |
| [14] |
Q.Q. Zhang, G.G. Ying, C.G. Pan, Y.S. Liu, J.L. Zhao, Environ. Sci. Technol. 49 (2015) 6772-6782. DOI:10.1021/acs.est.5b00729 |
| [15] |
J. Luis Martinez, Environ. Pollut. 157 (2009) 2893-2902. DOI:10.1016/j.envpol.2009.05.051 |
| [16] |
S.M. Sun, D.K.A. Korheina, H.T. Fu, X.P. Ge, Sci. Total Environ. 720 (2020) 137478. DOI:10.1016/j.scitotenv.2020.137478 |
| [17] |
J.F. Yan, J.L. Peng, L.D. Lai, et al., Environ. Sci. Technol. 52 (2018) 14302-14310. DOI:10.1021/acs.est.8b03340 |
| [18] |
H.X. Wang, N. Wang, J.H. Qian, et al., Environ. Sci. Technol. 51 (2017) 3518-3525. DOI:10.1021/acs.est.6b06474 |
| [19] |
H.X. Wang, N. Wang, B. Wang, et al., Environ. Sci. Technol. 50 (2016) 2692-2699. DOI:10.1021/acs.est.5b05749 |
| [20] |
S.I. Kawano, H.Y. Hao, Y. Hashi, J.M. Lin, Chin. Chem. Lett. 26 (2015) 36-38. DOI:10.1016/j.cclet.2014.10.026 |
| [21] |
C.S. Wu, J.L. Zhang, Y.L. Qiao, Y.L. Wang, Z.R. Chen, Chin. Chem. Lett. 22 (2011) 334-337. DOI:10.1016/j.cclet.2010.09.035 |
| [22] |
X.A. Ton, V. Acha, K. Haupt, B.T.S. Bui, Biosens. Bioelectron. 36 (2012) 22-28. DOI:10.1016/j.bios.2012.03.033 |
| [23] |
Y. Zhang, Y. Zhang, Y. Zhang, et al., Chin. Chem. Lett. 29 (2018) 1383-1386. DOI:10.1016/j.cclet.2017.10.026 |
| [24] |
A.V. Herrera-Herrera, L.M. Ravelo-Perez, J. Hernandez-Borges, et al., J. Chromatogr. A 1218 (2011) 5352-5361. DOI:10.1016/j.chroma.2011.06.031 |
| [25] |
M. Kamruzzaman, A.M. Alam, S.H. Lee, et al., J. Lumin. Appl. 132 (2012) 3053-3057. DOI:10.1016/j.jlumin.2012.06.013 |
| [26] |
A. Joshi, K.H. Kim, Biosens. Bioelectron. 153 (2020) 112046. DOI:10.1016/j.bios.2020.112046 |
| [27] |
L.Y. Lan, Y. Yao, J.F. Ping, Y.B. Ying, Biosens. Bioelectron. 91 (2017) 504-514. DOI:10.1016/j.bios.2017.01.007 |
| [28] |
Y.Y. Lu, X.Q. Liang, C. Niyungeko, et al., Talanta 178 (2018) 324-338. DOI:10.1016/j.talanta.2017.08.033 |
| [29] |
X.G. Liu, D.L. Huang, C. Lai, et al., Trac-TrendsAnal. Chem. 109 (2018) 260-274. DOI:10.1016/j.trac.2018.10.011 |
| [30] |
H. Aleixo, L.L. Okumura, A. Gurgel, A.F.S. Silva, J.A. Diniz, Anal. Methods 11 (2019) 1743-1750. DOI:10.1039/C9AY00094A |
| [31] |
C.S. Gondim, G.M. Duran, A.M. Contento, A. Rios, Food Anal. Methods 11 (2018) 1711-1721. DOI:10.1007/s12161-018-1157-6 |
| [32] |
M. Jacobs, V.J. Nagaraj, T. Mertz, et al., Anal. Methods 5 (2013) 4325-4329. DOI:10.1039/c3ay40994e |
| [33] |
P. Balasubramanian, R. Settu, S.M. Chen, T.W. Chen, Microchim. Acta 185 (2018) 396. DOI:10.1007/s00604-018-2934-z |
| [34] |
G.R. Zhou, J.H. Pan, Anal. Chim. Acta 307 (1995) 49-53. DOI:10.1016/0003-2670(95)00028-X |
| [35] |
G.V.S. Reddy, S.J. Reddy, Talanta 44 (1997) 627-631. DOI:10.1016/S0039-9140(96)02081-4 |
| [36] |
M. Rizk, F. Belal, F.A. Aly, N.M. El-Enany, Talanta 46 (1998) 83-89. DOI:10.1016/S0039-9140(97)00249-X |
| [37] |
A. Ambrosi, R. Antiochia, L. Campanella, R. Dragone, I. Lavagnini, J. Hazard. Mater. 122 (2005) 219-225. DOI:10.1016/j.jhazmat.2005.03.011 |
| [38] |
A. Radi, Z. El-Sherif, Talanta 58 (2002) 319-324. DOI:10.1016/S0039-9140(02)00245-X |
| [39] |
M.M. Ghoneim, A. Radi, A.M. Beltagi, J. Pharm. Biomed. Anal. 25 (2001) 205-210. DOI:10.1016/S0731-7085(00)00475-1 |
| [40] |
T. Gan, Z.X. Shi, J.Y. Sun, Y.M. Liu, Talanta 121 (2014) 187-193. DOI:10.1016/j.talanta.2014.01.002 |
| [41] |
J.Y. Sun, T. Gan, H.J. Zhu, Z.X. Shi, Y.M. Liu, Appl. Clay Sci. 101 (2014) 598-603. DOI:10.1016/j.clay.2014.09.025 |
| [42] |
P.P. Zhang, N.N. Zhang, L.J. Jing, et al., Int. J. Electrochem. Sci. 14 (2019) 9337-9346. |
| [43] |
S. Biswas, H. Naskar, S. Pradhan, et al., New J. Chem. 44 (2020) 1921-1930. DOI:10.1039/C9NJ04446A |
| [44] |
Z. Rouhbakhsh, A. Verdian, G. Rajabzadeh, Talanta 206 (2020) 120246. DOI:10.1016/j.talanta.2019.120246 |
| [45] |
Y.L. Sun, Y.X. Dai, X.D. Zhu, et al., Microchim. Acta 187 (2020) 63. DOI:10.1007/s00604-019-4012-6 |
| [46] |
F.H. Wu, F. Xu, L. Chen, et al., Chem. Res. Chin. Univ. 32 (2016) 468-473. DOI:10.1007/s40242-016-5367-4 |
| [47] |
K. Chullasat, P. Kanatharana, W. Limbut, A. Numnuam, P. Thavarungkul, Biosens. Bioelectron. 26 (2011) 4571-4578. DOI:10.1016/j.bios.2011.05.029 |
| [48] |
L.M. Feng, Q. Xue, F. Liu, et al., Microchim. Acta 187 (2020) 86. DOI:10.1007/s00604-019-4065-6 |
| [49] |
T. Chen, Y.R. Liu, J.H. Lu, et al., New J. Chem. 43 (2019) 15169-15176. DOI:10.1039/C9NJ03511G |
| [50] |
T.W. Chen, A.S. Vasantha, S.M. Chen, et al., Ultrason. Sonochem. 59 (2019) 104718. DOI:10.1016/j.ultsonch.2019.104718 |
| [51] |
S. Bonyadi, K. Ghanbari, M. Ghiasi, New J. Chem. 44 (2020) 3412-3424. DOI:10.1039/C9NJ05954G |
| [52] |
N. Sharma, S.P. Selvam, K. Yun, Appl. Surf. Sci. 512 (2020) 145742. DOI:10.1016/j.apsusc.2020.145742 |
| [53] |
M. Yadav, V. Ganesan, R. Gupta, D.K. Yadav, P.K. Sonkar, Microchem. J. 146 (2019) 881-887. DOI:10.1016/j.microc.2019.02.025 |
| [54] |
Z.P. Liu, M.L. Jin, H. Lu, et al., Sens. Actuator. B: Chem. 288 (2019) 363-372. DOI:10.1016/j.snb.2019.02.097 |
| [55] |
C. Fenzl, P. Nayak, T. Hirsch, et al., ACS Sens. 2 (2017) 616-620. DOI:10.1021/acssensors.7b00066 |
| [56] |
K. Starzec, C. Cristea, M. Tertis, et al., Bioelectrochemistry 132 (2020) 107405. DOI:10.1016/j.bioelechem.2019.107405 |
| [57] |
Y.Q. Zhu, C.K. Li, L.M. Wang, et al., Electroanalysis 31 (2019) 1446-1453. |
| [58] |
L. Fotouhi, M. Alahyari, Colloid Surf. B: Biointerfaces 81 (2010) 110-114. DOI:10.1016/j.colsurfb.2010.06.030 |
| [59] |
T.S. Chen, K.L. Huang, J.L. Chen, Bull. Environ. Contam. Toxicol. 89 (2012) 1284-1288. DOI:10.1007/s00128-012-0833-2 |
| [60] |
W. Wen, D.M. Zhao, X.H. Zhang, et al., Sens. Actuator. B: Chem. 174 (2012) 202-209. DOI:10.1016/j.snb.2012.08.010 |
| [61] |
X. Zhang, Y.L. Wei, Y.P. Ding, Anal. Chim. Acta 835 (2014) 29-36. DOI:10.1016/j.aca.2014.05.020 |
| [62] |
S. Liu, G.S. Lai, H.L. Zhang, A.M. Yu, Microchim. Acta 184 (2017) 1445-1451. DOI:10.1007/s00604-017-2138-y |
| [63] |
S. Jafari, M. Dehghani, N. Nasirizadeh, M. Azimzadeh, J. Electroanal. Chem. 829 (2018) 27-34. DOI:10.1016/j.jelechem.2018.09.053 |
| [64] |
Z.M. Jiang, Q. Liu, Y.R. Tang, M.X. Zhang, Electroanalysis 29 (2017) 602-608. DOI:10.1002/elan.201600408 |
| [65] |
F.D. Munteanu, A.M. Titoiu, J.L. Marty, A. Vasilescu, Sensors 18 (2018) 901-926. DOI:10.3390/s18030901 |
| [66] |
A. Veseli, F. Mullallari, F. Balidemaj, et al., Microchem. J. 148 (2019) 412-418. DOI:10.1016/j.microc.2019.04.086 |
| [67] |
A.M. Santos, A. Wong, F.C. Vicentini, O. Fatibello, Microchim. Acta 186 (2019) 174. DOI:10.1007/s00604-019-3296-x |
| [68] |
S. Jahanbani, A. Benvidi, Biosens. Bioelectron. 85 (2016) 553-562. DOI:10.1016/j.bios.2016.05.052 |
| [69] |
M. Elfiky, N. Salahuddin, A. Hassanein, A. Matsuda, T. Hattori, Microchem. J. 146 (2019) 170-177. DOI:10.1016/j.microc.2018.12.034 |
| [70] |
J.J. Dang, H. Cui, X.J. Li, J.L. Zhang, Anal. Sci. 35 (2019) 979-985. DOI:10.2116/analsci.19P127 |
| [71] |
A.H. Schebeliski, D. Lima, L. Marchesi, C.M.F. Calixto, C.A. Pessoa, J. Appl. Electrochem. 48 (2018) 471-485. DOI:10.1007/s10800-018-1171-9 |
| [72] |
A. Munawar, M.A. Tahir, A. Shaheen, et al., J. Hazard. Mater. 342 (2018) 96-106. DOI:10.1016/j.jhazmat.2017.08.014 |
| [73] |
A. Yari, A. Shams, Anal. Chim. Acta 1039 (2018) 51-58. DOI:10.1016/j.aca.2018.07.061 |
| [74] |
Z.P. Liu, M.L. Jin, J.P. Cao, et al., Sens. Actuator. B: Chem. 257 (2018) 1065-1075. DOI:10.1016/j.snb.2017.11.052 |
| [75] |
Y. Yuan, X.Z. Xu, J.F. Xia, et al., Microchim. Acta 186 (2019) 191. DOI:10.1007/s00604-019-3298-8 |
| [76] |
L.V. Faria, J.F.S. Pereira, G.C. Azevedo, et al., J. Braz. Chem. Soc. 30 (2019) 1947-1954. |
| [77] |
B.S. He, X.H. Yan, Sensors 18 (2018) 864-876. DOI:10.3390/s18030864 |
| [78] |
C. Chen, C. Chen, Y.T. Hong, T.W. Lee, J.F. Huang, Chem. Eng. J. 352 (2018) 188-197. DOI:10.1016/j.cej.2018.06.110 |
| [79] |
A. Wong, T.A. Silva, F.C. Vicentini, O. Fatibello, Talanta 161 (2016) 333-341. DOI:10.1016/j.talanta.2016.08.035 |
| [80] |
J.L. Song, M.H. Huang, N. Jiang, et al., J. Hazard. Mater. 391 (2020) 122024. DOI:10.1016/j.jhazmat.2020.122024 |
| [81] |
M. Roushani, Z. Rahmati, S. Farokhi, S.J. Hoseini, R.H. Fath, Mater. Sci. Eng. C: Mater. Biol. Appl. 108 (2020) 110388. DOI:10.1016/j.msec.2019.110388 |
| [82] |
W.W. Yi, Z.P. Li, C. Dong, H.W. Li, J.F. Li, Microchem. J. 148 (2019) 774-783. DOI:10.1016/j.microc.2019.05.049 |
| [83] |
N. Ajami, N.B. Panah, I. Danaee, Iran. Polym. J. 23 (2014) 121-126. DOI:10.1007/s13726-013-0207-6 |
| [84] |
L.L. Li, X.Q. Liu, L.W. Yang, et al., Biosens. Bioelectron. 142 (2019) 111525. DOI:10.1016/j.bios.2019.111525 |
| [85] |
T.S.H. Pham, P.J. Mahon, G. Lai, A. Yu, Electroanalysis 30 (2018) 2185-2194. DOI:10.1002/elan.201700738 |
| [86] |
S. Pilehvar, C. Reinemann, F. Bottari, et al., Sens. Actuator. B: Chem. 240 (2017) 1024-1035. DOI:10.1016/j.snb.2016.09.075 |
| [87] |
G. Krepper, G.D. Pierini, M.F. Pistonesi, M.S. Di Nezio, Sens. Actuator. B: Chem. 241 (2017) 560-566. DOI:10.1016/j.snb.2016.10.125 |
| [88] |
N.R. Jalal, T. Madrakian, A. Afkhami, M. Ghamsari, J. Electroanal. Chem. 833 (2019) 281-289. DOI:10.1016/j.jelechem.2018.12.004 |
| [89] |
A.M. Santos, A. Wong, A.A. Almeida, O. Fatibello, Talanta 174 (2017) 610-618. DOI:10.1016/j.talanta.2017.06.040 |
| [90] |
S.S. Khaloo, S. Mozaff1ri, A. Barekat, F. Karimi, Micro NanoLett. 10 (2015) 561-566. |
| [91] |
A. Zamora-Galvez, A. Ait-Lahcen, L.A. Mercante, et al., Anal. Chem. 88 (2016) 3578-3584. DOI:10.1021/acs.analchem.5b04092 |
| [92] |
A. Wong, A.M. Santos, F.H. Cincotto, et al., Talanta 206 (2020) 120252. DOI:10.1016/j.talanta.2019.120252 |
| [93] |
A.M. Santos, A. Wong, F.H. Cincotto, F.C. Moraes, O. Fatibello, Microchim. Acta 186 (2019) 148. DOI:10.1007/s00604-019-3268-1 |
| [94] |
S. Carvalho, T.B.S. Santana, C.R.S. Matos, et al., J. Braz. Chem. Soc. 30 (2019) 1266-1275. |
| [95] |
R.S. Lamarca, R.A. Dorledo de Faria, M.V. Boldrin Zanoni, et al., RSC Adv. 10 (2020) 1838-1847. DOI:10.1039/C9RA09083E |
| [96] |
Y.M. Guo, G.H. Shen, X. Sun, X.Y. Wang, IEEE Sens. J. 15 (2015) 1951-1958. DOI:10.1109/JSEN.2014.2370051 |
| [97] |
J. Ghodsi, A.A. Rafati, Y. Shoja, Sens. Actuator. B: Chem. 224 (2016) 692-699. DOI:10.1016/j.snb.2015.10.091 |
| [98] |
F. Conzuelo, S. Campuzano, M. Gamella, et al., Biosens. Bioelectron. 50 (2013) 100-105. DOI:10.1016/j.bios.2013.06.019 |
| [99] |
X.Y. He, H.M. Han, L.Q. Liu, et al., ACS Appl. Mater. Interfaces 11 (2019) 13676-13684. DOI:10.1021/acsami.9b00277 |
| [100] |
X.P. Hong, J.Y. Ma, Chin. Chem. Lett. 24 (2013) 329-331. DOI:10.1016/j.cclet.2013.02.010 |
| [101] |
P. Wang, X.F. Fu, J. Li, et al., Chin. Chem. Lett. 22 (2011) 611-614. DOI:10.1016/j.cclet.2010.12.004 |
| [102] |
S. Kesavan, D.R. Kumar, Y.R. Lee, J.J. Shim, Sens. Actuator. B: Chem. 241 (2017) 455-465. DOI:10.1016/j.snb.2016.10.091 |
| [103] |
M.Z. Cai, L. Zhu, Y.P. Ding, et al., Mater. Sci. Eng. C-Mater. Biol. Appl. 32 (2012) 2623-2627. DOI:10.1016/j.msec.2012.08.017 |
| [104] |
F. Luan, Y. Wang, S. Zhang, et al., Analyst 145 (2020) 1943-1949. DOI:10.1039/C9AN02575H |
| [105] |
Z. Peng, X.J. Liu, W. Zhang, et al., Environ. Int. 134 (2020) 105398. |
| [106] |
R.G. Xing, Y.N. Li, B.W. Zhang, Chin. Chem. Lett. 28 (2017) 407-411. DOI:10.1016/j.cclet.2016.10.017 |
| [107] |
K.W. Chen, L.B. Chen, Y.Q. Chen, H. Bai, L. Li, J. Mater. Chem. 22 (2012) 20968-20976. DOI:10.1039/c2jm34816k |
| [108] |
J.C. Yoon, J.S. Lee, S.I. Kim, K.H. Kim, J.H. Jang, Sci. Rep. 3 (2013) 1788-1795. DOI:10.1038/srep01788 |
| [109] |
X. Zhang, Y.C. Zhang, J.W. Zhang, Talanta 161 (2016) 567-573. DOI:10.1016/j.talanta.2016.09.013 |
| [110] |
M. Parmeggiani, P. Zaccagnini, S. Stassi, et al., ACS Appl. Mater. Interfaces 11 (2019) 33221-33230. DOI:10.1021/acsami.9b10408 |
| [111] |
B.S. He, L. Wang, X.Z. Dong, et al., Food Chem. 300 (2019) 125179. DOI:10.1016/j.foodchem.2019.125179 |
| [112] |
A.G. Fa, F. Pignanelli, I. Lopez-Corral, et al., Trac-TrendsAnal. Chem. 121 (2019) 115673. DOI:10.1016/j.trac.2019.115673 |
| [113] |
M. Roushani, Z. Rahmati, S.J. Hoseini, R.H. Fath, Colloid Surf. B: Biointerfaces 183 (2019) 110451. DOI:10.1016/j.colsurfb.2019.110451 |
| [114] |
N. Sebastian, W.C. Yu, D. Balram, Inorg. Chem. Front. 6 (2019) 82-93. DOI:10.1039/C8QI01000E |
| [115] |
T. Bhengo, M. Moyo, M. Shumba, O.J. Okonkwo, New J. Chem. 42 (2018) 5014-5023. DOI:10.1039/C8NJ00129D |
| [116] |
Q.K. Chen, L. Chen, J.J. Qi, et al., Chin. Chem. Lett. 30 (2019) 1214-1218. DOI:10.1016/j.cclet.2019.03.002 |
| [117] |
M. Roushani, K. Ghanbari, Anal. Methods 10 (2018) 5197-5204. DOI:10.1039/C8AY01815D |
| [118] |
X.H. Que, B.Q. Liu, L.B. Fu, et al., Electroanalysis 25 (2013) 531-537. DOI:10.1002/elan.201200468 |
| [119] |
F. Conzuelo, M. Gamella, S. Campuzano, A. Julio Reviejo, J.M. Pingarron, Anal. Chim. Acta 737 (2012) 29-36. DOI:10.1016/j.aca.2012.05.051 |
| [120] |
Z.G. Yu, A.L. Sutlief, R.Y. Lai, Sens. Actuator. B: Chem. 258 (2018) 722-729. DOI:10.1016/j.snb.2017.11.193 |
| [121] |
H.Y. Chen, J. Wang, L. Meng, T. Yang, K. Jiao, Chin. Chem. Lett. 27 (2016) 231-234. DOI:10.1016/j.cclet.2015.09.018 |
| [122] |
K. Zarei, A. Khodadadi, Ecotox. Environ. Safe. 144 (2017) 171-177. DOI:10.1016/j.ecoenv.2017.06.030 |
| [123] |
K.J. Huang, X. Liu, W.Z. Xie, H.X. Yuan, Colloid Surf. B: Biointerfaces 64 (2008) 269-274. DOI:10.1016/j.colsurfb.2008.02.003 |
| [124] |
W.J. Lian, S. Liu, J.H. Yu, et al., Anal. Lett. 46 (2013) 1117-1131. DOI:10.1080/00032719.2012.751540 |
| [125] |
X. Liu, S. Zheng, Y.X. Hu, et al., Food Anal. Methods 9 (2016) 2972-2978. DOI:10.1007/s12161-016-0480-z |
| [126] |
X. Bai, C.D. Qin, X. Huang, Microchim. Acta 183 (2016) 2973-2981. DOI:10.1007/s00604-016-1945-x |
| [127] |
Y.F. Huang, X.C. Yan, L.H. Zhao, et al., Microchem. J. 150 (2019) 104179. DOI:10.1016/j.microc.2019.104179 |
| [128] |
H. da Silva, J.G. Pacheco, J.M.C.S. Magalhaes, S. Viswanathan, C. DelerueMatos, Biosens. Bioelectron. 52 (2014) 56-61. DOI:10.1016/j.bios.2013.08.035 |
| [129] |
J.L. Yin, W.J. Guo, X.L. Qin, et al., Sens. Actuator. B: Chem. 241 (2017) 151-159. DOI:10.1016/j.snb.2016.10.062 |
| [130] |
T. Liu, Q. Xue, J.B. Jia, et al., Phys. Chem. Chem. Phys. 21 (2019) 16282-16287. DOI:10.1039/C9CP03486B |
 2021, Vol. 32
2021, Vol. 32