Over the past two decades, metal-directed self-assembly of supramolecular architectures have been increasing, and became a hot topic and frontier field in supramolecular chemistry [1-8]. A variety of high-symmetric palladium(Ⅱ) supramolecular structures based on bi- and poly-dentate ligands, such as cage, sphere and zeolite structures, have been synthesized [9-12]. However, low-symmetric bowl-shaped or cavity-shaped metallacalixarenes formed via metal-directed self-assembly approaches are still challenging. These metallacalixarenes have been attracted considerable attention for their impressive structural and functional analogues of the organic host calixarenes [13-15]. The term "metallacalixarenes" was coined by Lippert as a special heterocalixarene, in which the CH2-bridging entity of the classical calixarene is replaced by a metal center, i.e., the first metal analogue of calix[4]arene self-assembled by cis-protected Pt(Ⅱ) complexes and pyrimidine ligand [16-18]. Imidazole ligands, which have multiple bonding modes and excellent anion binding capacities, have evolved over the recent years in architected structurally diverse and multifunctional metallacalixarenes [19-25]. In contrast to the organic imidazolium receptors [26-29], imidazole-based metal-assembled anion receptors have the advantage naturally for their water solubility, more anion binding sites and multivariate binding modes, such as C—H···anion hydrogen bonds, N-H···anion hydrogen bonds and anion-π effect [30]. For this reason, we have developed a series of positively charged water-soluble metallacalixarenes anion receptors through self-assembly approach, such as molecular bowls, crowns and baskets, which show promise in binding inorganic anions [27-31].
In previous work, we synthesized metallacalixarenes using di-benzo[d]imidazoles and di-naphthoimidazoles bridged by methyl-, phenyl- and pyridyl-groups respectively [29-31]. These complexes present [M2L2]4+-type boat-shape structure through C-Himidzole···anion hydrogen bond. However, all these ligands are synthesized through imidazoles rigid coupling with bridging group. Compare to the rigid ligands, flexible ligands are more versatile and can easier tailor their structures for use as anion receptors. Therefore, study of palladium(Ⅱ)-based metallacalixarenes using flexible imidazole-pyridine ligand is important for understanding the role of flexible ligand in the self-assembly and anion recognition.
Herein, we reported the design and synthesis of novel lyophobic [ML2]2+-type and [ML]2+-type metallacalixarenes through a series of flexible pyridine-bridged diimidazole ligands (L1-L3) coordinated with Pd(Ⅱ)-based components (M1 and M2) as shown in Scheme 1.
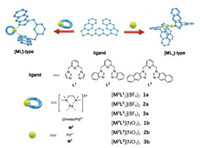
|
Download:
|
| Scheme 1. Self-assembly of the metallacalixarenes based on flexible pyridine-bridged imidazole ligand and un-protected palladium/cis-protected palladium building blocks. | |
Bidentate flexible pyridine-bridged diimidazole ligands L1-L3 were synthesized according to an established method [32]. After treating ligands with 0.5 equiv. of un-protected palladium building blocks M1-BF4 in DMSO for 2 h at 80 ℃ yielded the [ML2]2+-type double bowl-shape metallacalixarenes ([M1L12](BF4)2, 1a; [M1L22](BF4)2, 2a; [M1L32](BF4)2, 3a). On the other hand, mixing the equal equivalent of ligands and cis-protected palladium building blocks M2-NO3 in H2O for 2 h at 80 ℃ resulted in formation of the [ML]2+-type single bowl-shape metallacalixarenes ([M2L1](NO3)2, 1b; [M2L2](NO3)2, 2b; [M2L3](NO3)2, 3b). These complexes contained different anion (NO3−, BF4−, PF6−) were obtained by using corresponding silver salt. All complexes were characterized by NMR spectroscopy, mass spectrometry, elemental analysis and single crystal X-ray diffraction analysis. Notably, the [ML]2+-type metallacalixarenes are capable of reorganizing and sensing anions by multiple C—H···anion hydrogen bonds.
The signals of the protons on the complexes showed split and shift in comparison with those of the free ligands in the 1H NMR spectra. All the signals were assigned carefully based on coupling constants, integrals along with the corelation ships obtained from 1H-1H COSY or 1H-1H NOESY spectra (Figs. S12-S28 in Supporting information).
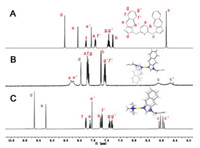
As shown in the NMR spectra of the [ML2]2+-type and [ML]2+-type metallacalixarenes (Fig. 1 and Figs. S12-S28), the protons on the methylene of the flexible ligand exhibit two broad peaks or two doublets, which show only a single peak in free ligand before self-assembly. This should be explained by that the CH2-protons of the flexible ligand in the complex are located in asymmetric chemical environment and demonstrated a highly symmetrical structure of the complex [29]. Meanwhile, the protons at Ce and Ce' had obvious shift toward downfield which show the deshielding effect of the palladium centre in the complex for both single and double bowl-shaped structures. Furthermore, for the single bowl-shape conformers, the proton signals at methyl in tmeda unit were split into two groups indicating that the methyl groups were in two different chemical environments. The highly symmetrical structures of the [ML2]2+-type and [ML]2+-type metallacalixarenes are further confirmed by the single-crystal X-ray diffraction analysis.
|
Download:
|
| Fig. 1. The expanded partial 1H NMR (400 MHz, DMSO-d6, 298 K) spectra of (A) ligand L3; (B) complex 3a-NO3; (C) complex 3b. | |
The identity and integrity of the [ML2]2+-type and [ML]2+-type metallacalixarenes in MeCN solution were confirmed by CSI and ESI mass spectrometry, which showed dominant signals at m/z = 290.33 for [1a-2BF4]2+, 392.00 for [2a-2BF4]2+, 492.00 for [3a-2BF4]2+, 523.1418 for [1b-NO3]+, 623.1732 for [2b-NO3]+, 724.2133 for [3b-NO3]+, with the expected isotopic distribution pattern for the dicationic or monocationic ions (Figs. S32-S37 in Supporting information).
Solid structural confirmation of [ML2]2+-type and [ML]2+-type metallacalixarenes were provided by X-ray crystallographic analysis (Figs. S38-S46 in Supporting information, CCDC Nos. 1886421, 1886422, 1886423 and 1919423 contain the crystallographic data of [M1L32](NO3)2 3a-NO3, [M1L32](PF6)2 (3a-PF6), [M1L22](NO3)2 (2a-NO3) and [M2L32](NO3)2, (3b), respectively). Selected bond distances and angles are listed in Tables S1- S9 in Supporting information.
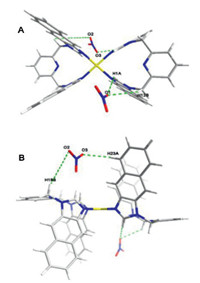
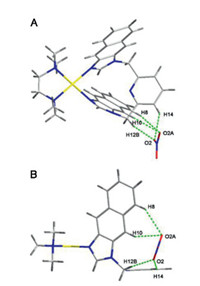
Structure of complex [M1L32](NO3)2 (3a-NO3) is depicted in Fig. 2 displaying [ML2]2+-type double bowl-shape metallacalixarene. Complex 3a-NO3 crystallized in the triclinic space group P-1. Central palladium ion is hosted in two bidentate compartments with the imidazole from two ligands, resulting in an overall {N4} coordination. Four Pd-Nimidazole distances all are 2.01 Å . The structure is stable because of the Nimidazole-Pd-Nimidazole bond angle are 85.77° and 94.23°, respectively, which are closed to right angle. The two pyridine rings in the complex were parallel. The dihedral angle between the naphthanoimidazolium plane and pyridine ring were 79.11° and 81.64°, respectively. The dihedral angle between the naphthanoimidazolium planes were 64.71° and 64.79°, respectively. As shown in Fig. S38, the 1D chain structure of 3a-NO3 is constructed through C—H···O hydrogen bonding interaction and π···π interaction between adjacent units. The NO3− ion acts as linker between two structural units. Structure of [M2L3](NO3)2 (3b) is shown in Fig. 3. The complex 3b crystallized in the orthorhombic space group Cmca. cis-Protected palladium building block coordinated with one ligand exhibiting [ML]2+-type single bowl-shape structure. The distances of Pd-Nimidazole are 2.04 Å and 2.06 Å . The structure is also stable because of the closed right angles of Nimidazole-Pd-Nimidazole (85.31°) and the Nimidazole-Pd-Ntmeda (94.20°). The dimer structure of the cation of 3b is architected through π···π interaction. As shown in Fig. S45B, the distance between pyridine rings from adjacent units was 3.61 Å , which is in the range of normal π···π interaction. Two crossed dimer were bridged via C—H···O hydrogen (O atom from NO3− ion) bonding interaction (Fig. S45C). Meanwhile, the crystal structures of 2a and 3a-PF6 are displayed in Figs. S40−44. The optimized structures of other [ML2]2+-type double bowl-shape and [ML]2+-type single bowl-shape complexes 1a, 1b and 2b were computed using the Scigress program (Version FJ 2.6; EU 3.1.8; Fujitsu Ltd., Krakow, Poland, 2014) and showed in Fig. S47 (Supporting information).
|
Download:
|
| Fig. 2. Crystal structure of complex [M1L32](NO3)2 (3a-NO3), top view (A) and side view (B). Hydrogen bonds show. | |
|
Download:
|
| Fig. 3. Crystal structure of complex [M2L3](NO3)2 (3b), top view (A) and side view (B). Hydrogen bonds show as green dashed line. Solvent molecules, non-hydrogen counter anions were omitted for clarity, yellow: Pd(Ⅱ); gray: C; blue: N; red: O. | |
as green dashed line. Solvent molecules, non-hydrogen counter anions are omitted for clarity, yellow: Pd(Ⅱ); gray: C; blue: N; red: O.
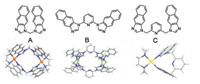
In our previous work, [M2L2]4+-type bowl-shape and boat-shape complexes were synthesized through self-assembly of cis-protected palladium unit [(tmeda)Pd(NO3)2] with diimidazole ligand di(1H-aphtho[2,3-d]imidazol-1-yl)methane and 2,6-bis(1H-naphtho[2,3-d]imidazol-1-yl) pyridine [29-31]. Compared to previous work, a novel [ML]2+-type complex was synthesized via flexible ligand coordinated with only one (tmeda)Pd(NO3)2 unit in this work (Fig. 4). The difference in these structures can be explained by the flexibility of ligand. The flexible ligand twist the naphtho[2,3-d]imidazole part to result in energy-efficient three-dimensional conformation for thermodynamic stability. This phenomenon illustrates that ligands' flexibility displays important influence in constructing the structure of complex.
|
Download:
|
| Fig. 4. Crystal structures of the complexes (top view) based on diimidazole ligands: di(1Hnaphtho[2,3-d]imidazol-1-yl)methane (A) [30], 2,6-bis(1H-naphtho[2,3-d]imidazol-1-yl) (B) [31] pyridine and L3 (C). | |
The anion binding properties of these palladium(Ⅱ)-based metallacalixarenes were investigated by NMR titration experiments in DMSO. No significant change was observed in the NMR spectra after the addition of 8 equiv. of tetrabutylammonium anion salt to a solution of [ML2]2+-type complexes with PF6− as counter anions (1a-PF6, 2a-PF6 and 3a-PF6) as shown in Figs. S48-S50 (Supporting information). On the contrary, upon addition of anions to a solution of [ML]2+-type complexes (1b-PF6, 2b-PF6 and 3b-PF6), significant downfield chemical shift of the protons in imidazole units were exhibited on the NMR timescale (Figs. S51–S65 in Supporting information). This could be explained by the property of structures of the two different types of metallacalixarenes. In the [ML2]2+-type complexes, the anion cannot affect the configuration of the monomer because the flexible ligand was locked by the central palladium atom. Meanwhile, the anion only affected the stacking structure of these [ML2]2+-type complexes through changing the arrangement mode between the monomer. In the [ML]2+-type complexes, the C-Himizole···anion hydrogen bond effect caused the particular NMR signal shift. Importantly, the job plots (Figs. S66–S80 in Supporting information) demonstrated the predominant host-guest species in these [ML]2+-type complexes were 1:1 stoichiometric complex. BindFit analysis of the titration data enabled the determination of the anion association constants shown in Table 1 [33]. The Ka of these receptors became greater with the increase in number of the hydrogen bond donor groups in the ligand units.
|
|
Table 1 Stability constants Ka (L/mol) for receptors 1b-PF6, 2b-PF6 and 3b-PF6 with tetrabutylammonium anion salts in DMSO-d6 at 298 K. |
In summary, a series of highly symmetrical palladium(Ⅱ)-based [ML]2+-type and [ML2]2+-type metallacalixarenes using the flexible pyridine-bridged diimidazole ligands were synthesized via coordination-driven self-assembly nearly in quantitative yields. Complexes were characterized both in the solid state (X-ray diffraction, element analysis) and in the solution (NMR, CSI-MS, ESI-HRMS). The flexibility of the ligands in these complexes plays a vital role in forming various configurations. At the same time, anions plays a vital role in regulation of the crystal architectures through multiple C—H···anion hydrogen bonds and other weak interactions. In addition, these [ML]2+-type metallacalixarenes are capable of reorganizing and sensing anions and are being used as anion receptor and sensor
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21906002), Beijing Municipal Science and Technology Project (No. KM202010005010), Beijing Municipal Natural Science Fund Project of Beijing Education Committee science and technology project key project (No. KZ201710005001), Beijing municipal high level innovative team building program (No. IDHT20180504) and Beijing Outstanding Young Scientist Program (No. BJJWZYJH01201910005017).
Appendix A. Supplementary dataSupplementarymaterial related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.053.
| [1] |
D.S. Lawrence, T. Jiang, M. Levett, Chem. Rev. 95 (1995) 2229-2260. DOI:10.1021/cr00038a018 |
| [2] |
L.F. Lindoy, I.M. Atkinson, Self-assembly in Supramolecular Systems. Cambridge: Royal Society of Chemistry, 2000.
|
| [3] |
J.A. McCleverty, T.J. Meyer, Comprehensive Coordination Chemistry Ⅱ, Elsevier, Amsterdam, 2003, pp. 369-393.
|
| [4] |
S.Y. Yu, S.H. Li, H.P. Huang, et al., Curr. Org. Chem. 9 (2005) 555-563. |
| [5] |
B.H. Northrop, Y.R. Zheng, K.W. Chi, P. Stang, J. Acc. Chem. Res. 42 (2009) 1554-1563. DOI:10.1021/ar900077c |
| [6] |
M.M.J. Smulders, I.A. Riddell, C. Browne, J.R. Nitschke, Chem. Soc. Rev. 42 (2013) 1728-1754. DOI:10.1039/C2CS35254K |
| [7] |
L. Xu, Y.X. Wang, L.J. Chen, H.B. Yang, Chem. Soc. Rev. 44 (2015) 2148-2167. DOI:10.1039/C5CS00022J |
| [8] |
S.Y. Yu, H.L. Lu, Isr. J. Chem. 59 (2019) 166-183. DOI:10.1002/ijch.201800097 |
| [9] |
R. Banerjee, A. Phan, B. Wang, et al., Science 319 (2008) 939-943. DOI:10.1126/science.1152516 |
| [10] |
Q.F. Sun, J. Wasa, D. Ogawa, et al., Science 328 (2010) 1144-1147. DOI:10.1126/science.1188605 |
| [11] |
N.B. Debata, D. Tripathy, D.K. Chand, Coord. Chem. Rev. 256 (2012) 1831-1945. DOI:10.1016/j.ccr.2012.04.001 |
| [12] |
M.L. Saha, X. Yan, P. Stang, J. Acc. Chem. Res. 49 (2016) 2527-2539. DOI:10.1021/acs.accounts.6b00416 |
| [13] |
C.D. Gutsche, Calixarenes Revisited. RSC, Cambridge, 1998.
|
| [14] |
J. Kuleszaa, B.S. Barros, S. Alves Júnior, Coord. Chem. Rev. 257 (2013) 2192-2212. DOI:10.1016/j.ccr.2013.03.031 |
| [15] |
P.D. Frischmann, M. MacLachlan, J. Chem. Soc. Rev. 42 (2013) 871-890. DOI:10.1039/C2CS35337G |
| [16] |
H. Rauter, E.C. Hillgeris, B. Lippert, J. Chem. Soc. Chem. Commun. (1992) 1385-1387. |
| [17] |
E.C. Hillgeris, A. Erxleben, B.J. Lippert, Am. Chem. Soc. 116 (1994) 616-624. DOI:10.1021/ja00081a023 |
| [18] |
B. Lippert, P. Miguel, J.S. Chem, Soc. Rev. 40 (2011) 4475-4487. DOI:10.1039/c1cs15090a |
| [19] |
R. Chakrabarty, P.S. Mukherjee, P.J. Stang, Chem. Rev. 111 (2011) 6810-6918. DOI:10.1021/cr200077m |
| [20] |
L.Y. Yao, L. Qin, T.Z. Xie, Y.Z. Li, S.Y. Yu, Inorg. Chem. 50 (2011) 6055-6062. DOI:10.1021/ic200047t |
| [21] |
D. Samanta, P.S. Mukherjee, Chem. Commun. 50 (2014) 1595-1598. DOI:10.1039/c3cc47965j |
| [22] |
B. Roy, A.K. Ghosh, S. Srivastava, P. D'Silva, P.S. Mukherjee, J. Am. Chem. Soc. 137 (2015) 11916-11919. DOI:10.1021/jacs.5b08008 |
| [23] |
D. Samanta, A. Chowdhury, P.S. Mukherjee, Inorg. Chem. 55 (2016) 1562-1568. DOI:10.1021/acs.inorgchem.5b02464 |
| [24] |
L.P. Zhou, Q.F. Sun, Chem. Commun. 51 (2015) 16767-16770. DOI:10.1039/C5CC07306E |
| [25] |
H. Dasary, R. Jagan, D.K. Chand, Inorg. Chem. 57 (2018) 12222-12231. DOI:10.1021/acs.inorgchem.8b01884 |
| [26] |
P. Sabater, F. Zapata, A. Caballero, et al., J. Org. Chem. 81 (2016) 3790-3798. DOI:10.1021/acs.joc.6b00468 |
| [27] |
S.Y. Yu, H. Huang, H.B. Liu, et al., Angew. Chem. Int. Ed. 42 (2003) 686-690. DOI:10.1002/anie.200390190 |
| [28] |
G.H. Ning, T.Z. Xie, Y.J. Pan, Y.Z. Li, S.Y. Yu, Dalton Trans. 39 (2010) 3203-3211. DOI:10.1039/b926138a |
| [29] |
T.Z. Xie, C. Guo, S.Y. Yu, Y.J. Pan, Angew. Chem. Int. Ed. 51 (2012) 1177-1181. DOI:10.1002/anie.201106504 |
| [30] |
W. Deng, Z.S. Yu, H.W. Ma, S.Y. Yu, Chem. Asian J. 13 (2018) 2805-2811. DOI:10.1002/asia.201800954 |
| [31] |
W. Deng, Z.S. Yu, X.H. Liu, S.Y. Yu, Chem. Asian J. 21 (2018) 3173-3179. |
| [32] |
C.R. Elie, G. David, A.R. Schmitzer, J. Med. Chem. 58 (2015) 2358-2366. DOI:10.1021/jm501979f |
| [33] |
 2021, Vol. 32
2021, Vol. 32 


