b School of Basic Medicine, Gannan Medical University, Ganzhou 341000, China;
c State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
Since the reagent of DABCO·(SO2)2 (1,4-diazabicyclo[2.2.2] octane-sulfur dioxide) developed by Santos and Mello [1] was firstly applied in 2010 by Willis [2] and potassium metabisulfite as the source of sulfur dixoide was reported in 2012 by Wu [3], the strategies with the insertion of sulfur dioxide under transition metal catalysis or radical processes have been developed rapidly [4]. Among the inorganic sulfites screened in the palladium-catalyzed reaction of aryl halides with hydrazines, potassium metabisulfite was demonstrated as the most efficient one [3]. Later, the development of radical process was boomed, and potassium or sodium metabisulfite was effective as well in the radical reactions [4a, 5]. So far, significant progress has been witnessed in this field of sulfur dioxide insertion. As a result, diverse sulfonyl-containing (-SO2-) compounds including sulfones and sulfonamides are generated easily by using DABCO·(SO2)2 and inorganic sulfites as the sulfur dioxide surrogates [6]. Compared with the classical synthesis of sulfonyl compounds from the oxidation of sulfides and sulfonylation with the corresponding sulfonyl chlorides, the incorporation of sulfonyl unit into small molecules through the insertion of sulfur dioxide provides an alternative and comple-mentary route. Besides the reagent of DABCO·(SO2)2 and inorganic sulfites (potassium or sodium metabisulfite), other sources are also explored for the generation of sulfonyl compounds. For example, Rongalite, commonly used as a bleaching agent in the printing and dyeing industry, was used as the source of sulfonyl group as well [7, 8]. Luo and co-workers described the iron-catalyzed reaction between rongalite and diaryliodonium salts [8a]. In this transformation, rongalite was used as the sulfoxylate anion radical precursor. Wu and co-workers reported the synthesis of N-aminosulfonamide through a metal-free reaction of rongalite with aryldiazonium tetrafluoroborates [8b]. During the reaction pro-cess, rongalite was found to serve as a radical initiator, a sulfur dioxide surrogate and a reducing reagent simultaneously.
Recently, sodium dithionite and thiourea dioxide have attracted our attention, which are cheap and easily available. It is reported that sodium dithionite (Na2S2O4) can be used as a reductant in various organic transformations [9]. Additionally, it can be used as the sulfoxylate anion radical precursor as well. In the iron-catalyzed reaction of rongalite and diaryliodonium salts as mentioned above [8a], sodium dithionite was also employed as a replacement of rongalite, although the yield was low. Since the commercially available and cheap sodium dithionite as sulfoxylate anion radical precursor could produce the SO2 anion radical, the application of sodium dithionite in the sulfonylation reactions could be expected.
In 2019, Wu and co-workers described the generation of sulfones and sulfonamides starting from (hetero)aryl iodides and sodium dithionite under catalyst-free conditions (Scheme 1) [10]. During the reaction process, sodium dithionite acted as a reductant and reacted with (hetero)aryl iodide leading to (hetero)aryl radical and sulfur dioxide. The (hetero)aryl radical would further combine with SO2 anion radical to provide the corresponding sodium sulfonate, which would be trapped by electrophile to furnish sulfones or sulfonamides in one pot under suitable conditions. The control experiments and several EPR experiments confirmed that (hetero)aryl radical intermediate would be produced from (hetero)aryl iodidein the presence of sulfur dioxide radical anion. The reaction conditions were mild, and a broad substrate scope with excellent functional group tolerance was observed. For instance, 2-sulfonated adenosine with free hydroxyl and amino groups was obtained in 56% yield.

|
Download:
|
| Scheme 1. Generation of sulfones and sulfonamides starting from (hetero)aryl iodides and sodium dithionite under catalyst-free conditions. | |
Later, Jiang and co-workers reported the construction of sterically bulky sulfones through a multicomponent decarboxylative reaction of N-hydroxyphthalimide esters, sodium dithionite, and electrophiles (Scheme 2) [11]. In this transformation, sodium dithionite served as the radical initiator for the decarboxylation as well as the sulfonyl source. It was noteworthy that the reactions of diverse naturally abundant carboxylic acids and other carboxyl-containing drugs with multiple heteroatoms and sensitive functional groups proceeded smoothly with decarboxylative sulfonylation, leading to sterically bulky tertiary sulfones in moderate to good yields. The mechanistic studies were performed, which revealed that sulfur dioxide radical anion generated from sodium dithionite would undergo a single electron transfer (SET) process with carboxylic-derived redox-active ester assisted by ZnCl2, giving rise to sterically hindered carbon radical with the extrusion of CO2. This sterically hindered carbon radical intermediate would react with sulfur dioxide radical anion to produce sulfinate salt, which would be trapped by electrophile leading to the corresponding sulfone.
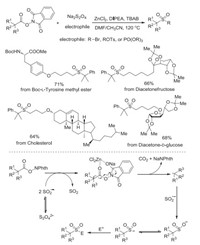
|
Download:
|
| Scheme 2. Multicomponent decarboxylative reaction of N-hydroxyphthalimide esters, sodium dithionite, and electrophiles. | |
In Wu's report [10], the three-component reaction of iodo-benzene, sodium dithionite, and alkyl bromide gave a low yield (18%). Jiang and co-workers described an improved procedure by using palladium catalyst in the reaction system (Scheme 3) [12]. In this transformation, phosphate esters were used as the electro-philes to capture the sulfinate intermediate. The addition of palladium catalyst would be beneficial for the final outcome. Initially, an oxidative addition of Pd(0) with aryl iodide would take place leading to Pd(Ⅱ) complex, which would undergo a single electron transfer with sulfur dioxide radical anion to provide another Pd(Ⅱ) complex. Further reductive elimination would occur to produce the corresponding sulfinate intermediate.
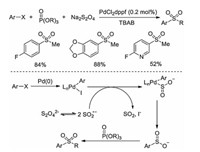
|
Download:
|
| Scheme 3. Palladium-catalyzed reaction of (hetero)aryl iodides, sodium dithionite and phosphate esters. | |
Thiourea dioxide is a good alternative as well for the introduction of sulfonyl group into small molecules, since thiourea dioxide would be converted to sulfur dioxide anion and urea in the presence of base [13]. Wu and co-workers disclosed the generation of heteroaryl sulfones and sulfonamides from heteroaryl halides and thiourea dioxide in the presence of sodium hydroxide under visible light irradiation (Scheme 4) [14]. This transformation also went through heteroaryl sulfinate intermediates, which were trapped subsequently by various electrophiles. The conditions were suitable for diverse substrates, especially for the electrondeficient heteroaryl halides. Control experiments showed that heteroaryl radical generated in situ would couple with sulfur dioxide radical anion during the reaction process with the assistance of photoredox catalyst, as supported by EPR spectros-copy and DFT calculations.
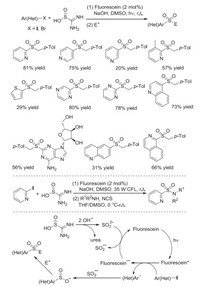
|
Download:
|
| Scheme 4. Synthesis of heteroaryl sulfones and sulfonamides from heteroaryl halides and thiourea dioxide under visible light irradiation. | |
Wu and co-workers further developed the sulfonylation of alkenes through photoredox-catalyzed functionalization of alkenes with thiourea dioxide under visible-light irradiation (Scheme 5) [15]. Alkyl sulfones and sulfonamides could be easily accessed through a reaction of alkenes, thiourea dioxide and electrophiles withbroad substrate scope.Good functional group compatibility and excellent regioselectivity were observed. A plausible mechanism supported by fluorescence quenching experiments was proposed, involving a radical addition process with sulfur dioxide radical anion (SO2·-) derived from the oxidation of sulfur dioxide anion (SO22-) under visible-light irradiation.
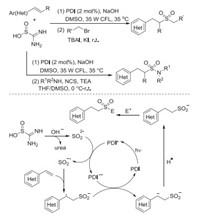
|
Download:
|
| Scheme 5. Photoredox-catalyzed functionalization of alkenes with thiourea dioxide under visible-light irradiation. | |
Followed by Wu's report [14, 15], Jiang and co-workers described the synthesis of sulfones from alkyl bromide, thiourea dioxide and phosphate esters (Scheme 6) [12]. Again, thiourea dioxide was used as a reductive sulfur dioxide surrogate, and the alkylsulfones were obtained in moderate to good yields. A similar reaction pathway was proposed, which showed that the sulfur dioxide radical anion formed from thiourea dioxide in the presence of caesium carbonate would initiate the reaction. The resulting alkyl sulfinate salt would be captured by phosphate ester, giving rise to the desired alkylsulfone.
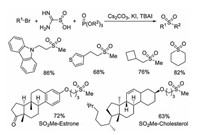
|
Download:
|
| Scheme 6. Synthesis of sulfones from alkyl bromide, thiourea dioxide and phosphate esters. | |
In conclusion, the cheap and easily available sodium dithionite and thiourea dioxide have been used as the source of sulfonyl group in the synthesis of sulfones and sulfonamides recently. Compared with other methods for the sulfonylation reactions, the strategies using sodium dithionite or thiourea dioxide provide an alternative and complementary route to diverse sulfonyl compounds. During the reaction process, sulfur dioxide anion radical is the key intermediate, which is usually generated from a single electron transfer under suitable conditions. The advantages using sodium dithionite or thiourea dioxide in the sulfonylation reactions include mild conditions and broad substrate scope with excellent functional group compatibility. Further applications by using sodium dithionite and thiourea dioxide in organic transformations will be anticipated.
Declaration of competing interestThe authors declare that there are no conflicts of interest.
AcknowledgmentsFinancial support from the National Natural Science Foundation of China (Nos. 21871053 and 21532001) and the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (No. 2019R01005) is gratefully acknowledged.
| [1] |
P.S. Santos, M.T.S. Mello, J. Mol. Struc. 178 (1988) 121-133. DOI:10.1016/0022-2860(88)85010-5 |
| [2] |
B. Nguyen, E.J. Emmet, M.C. Willis, J. Am. Chem. Soc. 132 (2010) 16372-16373. DOI:10.1021/ja1081124 |
| [3] |
S. Ye, J. Wu, Chem. Commun. 48 (2012) 10037-10039. DOI:10.1039/c2cc34957d |
| [4] |
(a) G. Qiu, K. Zhou, L. Gao, J. Wu, Org. Chem. Front. 5 (2018) 691-705; (b) K. Hofman, N. Liu, G. Manolikakes, Chem. Eur. J. 24 (2018) 11852-11863; (c) D. Zheng, J. Wu, Sulfur Dioxide Insertion Reactions for Organic Synthesis, Nature Springer, Berlin, 2017; (d) G. Liu, C. Fan, J. Wu, Org. Biomol. Chem. 13 (2015) 1592-1599; (e) E.J. Emmett, M.C. Willis, Asian J. Org. Chem. 4 (2015) 602-611; (f) A.S. Deeming, E.J. Emmett, C.S. Richards-Taylor, M.C. Willis, Synthesis 46 (2014) 2701-2710; (g) P. Bisseret, N. Blanchard, Org. Biomol. Chem. 11 (2013) 5393-5398; (h) G. Qiu, L. Lai, J. Cheng, J. Wu, Chem. Commun. 54 (2018) 10405-10414; (i) G. Qiu, K. Zhou, J. Wu, Chem. Commun. 54 (2018) 12561-12569; (j) S. Ye, G. Qiu, J. Wu, Chem. Commun. 55 (2019) 1013-1019; (k) S. Ye, M. Yang, J. Wu, Chem. Commun. 56 (2020) 4145-4155; (l) M. Wang, X. Jiang, Chin. Sci. Bull. 63 (2018) 2707-2716. |
| [5] |
(a) D. Zheng, Y. An, Z. Li, J. Wu, Angew. Chem. Int. Ed. 53 (2014) 2451-2454; (b) D. Zheng, J. Yu, J. Wu, Angew. Chem. Int. Ed. 55 (2016) 11925-11929. |
| [6] |
(a) Z. Chen, N.W. Liu, M. Bolte, H. Ren, G. Manolikakes, Green Chem. 20 (2018) 3059-3070; (b) Q. Lin, Y. Liu, Z. Xiao, et al., Org. Chem. Front. 6 (2019) 447-450; (c) X.Y. Qin, L. He, J. Li, et al., Chem. Commun. 55 (2019) 3227-3230; (d) R.J. Reddy, A.H. Kumari, J.J. Kumar, J.B. Nanubolu, Adv. Synth. Catal. 361 (2019) 1587-1591; (e) L. Lu, C. Luo, H. Peng, et al., Org. Lett. 21 (2019) 2602-2605; (f) S. Ye, D. Zheng, J. Wu, G. Qiu, Chem. Commun. 55 (2019) 2214-2217; (g) J. Zhang, W. Xie, S. Ye, J. Wu, Org. Chem. Front. 6 (2019) 2254-2259; (h) Y. Chen, P.R. D. Murray, A.T. Davies, M.C. Willis, J. Am. Chem. Soc. 140 (2018) 8781-8787; (i) B. Ni, B. Zhang, J. Han, et al., Org. Lett. 22 (2020) 670-674; (j) K. Zhou, J.B. Liu, W. Xie, S. Ye, J. Wu, Chem. Commun. 56 (2020) 2554-2557; (k) J. Zhang, M. Yang, J.B. Liu, F.S. He, J. Wu, Chem. Commun. 56 (2020) 3225-3228; (l) X. Gong, M. Yang, J.B. Liu, et al., Green Chem. 22 (2020) 1906-1910; (m) S. Ye, K. Zhou, P. Rojsitthisak, J. Wu, Org. Chem. Front. 7 (2020) 14-18; (n) K. Chen, W. Chen, B. Han, et al., Org. Lett. 22 (2020) 1841-1845. |
| [7] |
S. Kotha, P. Khedkar, Chem. Rev. 112 (2012) 1650-1680. DOI:10.1021/cr100175t |
| [8] |
(a) W. Zhang, M. Luo, Chem. Commun. 52 (2016) 2980-2983; (b) M. Wang, B.C. Tang, J.G. Wang, et al., Chem. Commun. 54 (2018) 7641-7644; (c) A. Shavnya, S.B. Coffey, K.D. Hesp, S.C. Ross, A.S. Tsai, Org. Lett. 18 (2016) 5848-5851. |
| [9] |
(a) Z.Y. Long, Q.Y. Chen, J. Org. Chem. 64 (1999) 4775-4782; (b) M.O. Ratnikov, D.L. Lipilin, A.M. Churakov, Y.A. Strelenko, V.A. Tartakovsky, Org. Lett. 4 (2002) 3227-3229; (c) H.J. Xu, Y.C. Liu, Y. Fu, Y.D. Wu, Org. Lett. 8 (2006) 3449-3451; (d) P. Cao, C.Y. Li, Y.B. Kang, et al., J. Org. Chem. 72 (2007) 6628-6630; (e) S. Oda, H. Shimizu, Y. Aoyama, et al., Org. Process Res. Dev. 16 (2012) 96-101; (f) I.S. Kondratov, M.Y. Bugera, N.A. Tolmachova, et al., J. Org. Chem. 80 (2015) 12258-12264. |
| [10] |
Y. Li, T. Liu, G. Qiu, J. Wu, Adv. Synth. Catal. 361 (2019) 1154-1159. DOI:10.1002/adsc.201801445 |
| [11] |
Y. Li, S. Chen, M. Wang, X. Jiang, Angew. Chem. Int. Ed. (2020). DOI:10.1002/anie.202001589 |
| [12] |
S. Chen, Y. Li, M. Wang, X. Jiang, Green Chem. 22 (2020) 322-326. DOI:10.1039/C9GC03841H |
| [13] |
(a) E.B. Barnett, J. Chem. Soc. 97 (1910) 63-65; (b) K. Nakagawa, K. Minami, Tetrahedron Lett. 13 (1972) 343-346; (c) R.C. L. Mangoni, G. Palumbo, L. Previtera, Tetrahedron Lett. (1975) 1041-1042; (d) R.B. dos Santos, T.J. Brocksom, U. Brocksom, Tetrahedron Lett. 38 (1997) 745-748. |
| [14] |
S. Ye, Y. Li, J. Wu, Z. Li, Chem. Commun. 55 (2019) 2489-2492. DOI:10.1039/C9CC00008A |
| [15] |
Y. Li, J.B. Liu, F.S. He, J. Wu, Chin. J. Chem. 38 (2020) 361-366. DOI:10.1002/cjoc.201900505 |
 2021, Vol. 32
2021, Vol. 32 

