b Department of Forensic Science, Gannan Medical University, Ganzhou 341000, China;
c School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China;
d College of Biological, Chemical Sciences and Engineering, Jiaxing University, Jiaxing 314001, China
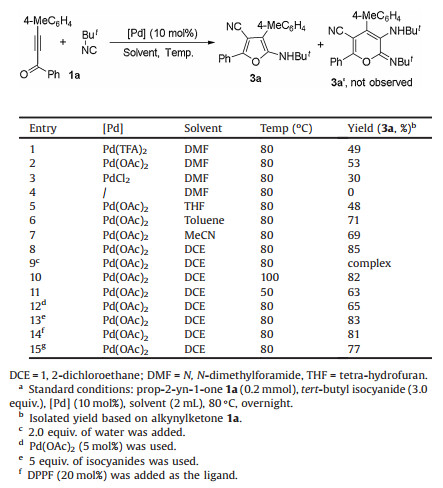
Isocyanides-based chemistry, highlighted by Passerini reaction and Ugi reaction, has attracted ever-increasing attention of synthetic chemists [1]. In the past decades, isocyanides have been well-recognized as a valuable C1 synthons [2-4]. As an important protocol to prepare cyclic products from acyclic substrates, cycloaddition was a type of history-long reaction [5]. In consideration of synthetic versatility of isocyanides, isocyanides-based cycloaddition (imidoylative cycloaddition) was a topic deserved to be exploited [6]. To the best of our knowledge, most of imidoylative cycloaddition often required using a stoichiometric loading of promoters [6, 7].
In 2003, a catalytic version was initiated by Chatani and co-workers with a [4 + 1] cycloaddition of α, β-unsaturated carbonyl compounds with isocyanides [8]. Based on their findings, the use of GaCl3, a catalyst with low affinity towards oxygen and nitrogen atoms, enabled the catalytic imidoylative [4 + 1] cycloaddition with high reaction efficiency and a broad reaction scope (Scheme 1a). Subsequently, the results from Zhu and co-workers suggested [4 + 1] cycloaddition of α, β-unsaturated imines with isocyanides also worked well in the presence of a catalytic amount of AlCl3 [9]. Recently, Jia, Li and co-workers reported an ytterbium and silver co-catalyzed synthesis of pyrrole-fused bicycles from enynones and isocyanides [10]. In the process, a catalytic imidoylative stepwise [4+1+1] cycloaddition enabled by Yb(OTf)3 was witnessed (Scheme 1b).
|
Download:
|
| Scheme 1. Proposed route for the synthesis of furan derivatives through palladium-catalyzed cyclization of prop-2-yn-1-ones and isocyanides. | |
On the other hand, palladium chemistry is a powerful tool to construct carbon-carbon bond and carbon-heteroatom bond [11]. Palladium catalysis was often used in isocyanides-based transformation, partially because of its easy coordination and ligand dissociation of palladium complexes with isocyanides. To our surprise, it remains rare that cycloaddition of 1, 3-conjugated system with isocyanides was promoted by palladium catalyst [12]. Theoretically, more synthetic versatilities of isocynides might be developed since palladium often served as Lewis acid catalyst and transitional metal catalyst as well. Very recently, a first palladium-catalyzed [4 + 1] cycloaddition of prop-2-yn-1-imines with isocyanides was established by our group for the synthesis of polysubstituted pyrroles (Scheme 1c) [12]. In this reaction, double isocyanides were incorporated as expected. Interestingly, one of isocyanides was installed as imidoyl group, and another was converted into cyano group. Moreover, it is noteworthy that the palladium-catalyzed [4 + 1] cycloaddition of prop-2-yn-1-imines with isocyanides was a concerted pathway, which was distinctive from the previous findings.
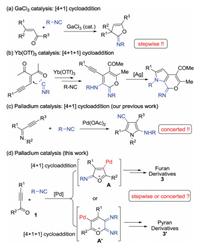
Inspired what mentioned above together with our continuous interest in regioselective transformation of alkynes [13], we would like to disclose imidoylative cycloaddition of prop-2-yn-1-ones under palladium catalysis (Scheme 1d). In the process, it was hypothesized that a palladium-catalyzed cycloaddition with isocyanides happened to prop-2-yn-1-ones 1 to produce furan cation-containing palladium species A or pyran cation-containing palladium species A′. The proposed key intermediates A and A′ were probably trapped by another isocyanides to offer polysubstituted furan derivatives 3 or polysubstituted pyran derivates 3′. To verify the possibility of this projected transformation, we started to optimize the reaction conditions.
The reaction of 1-phenyl-3-(p-tolyl)prop-2-yn-1-one 1a and tert-butyl isocyanides 2a was employed as a model reaction. Initial trials were focused on screening various palladium sources. As presented in Table 1, Pd(OAc)2 was the best choice, leading to the desired 2-amino-4-cyanofuran 3a in a 53% yield (entry 2) as expected. The [4 + 1+1] cycloaddition pyran product 3a′, dominated in Jia and Li's results, was not afforded at all. Considering high importance of 2-amino-4-cyanofuran [14], we further optimize the reaction.
|
|
Table 1 Initial studies for the reaction of [4 + 1] cycloaddition of prop-2-yn-1-ones and isocyanides under palladium catalysis.a |
When Pd(TFA)2 and PdCl2 were used as catalysts (entries 1 and 3), inferior outcomes towards 3a were observed. A blank reaction without palladium catalyst was totally retarded, showing the high importance of palladium catalyst in the process (entry 4). Then, solvent effect was also explored accordingly. From the results, moisture in solvent made a significant impact on the reaction efficiency. The use of DCE, with relatively poor water solubility, gave rise to the desired product 3a in 85% yield (entry 8). No better yields were detected when using THF, toluene and MeCN as solvents (entries 5–7). To prove this assumption, the model reaction at DCE with 2 equiv. of water as additive was conducted. The control experiment became complex and did not provided the desired product 3a (entry 9). Increase or decrease of reaction temperature did not improve the reaction efficiency (entries 10 and 11). The reaction was greatly supressed when the loading of palladium was reduced to 5 mol% (entry 12). To increase isocyanides loading to 5.0 equiv., the yield toward the desired product 3a was not improved, and moreover, the [4+1+1] cycloaddition product 3a′ was still not observed. This above result probably suggested palladium-catalyzed cycloaddition herein underwent a distinctive pathway from that of GaCl3 catalysis and Yb(OTf)3 catalysis. Supposing the cycloaddition of prop-2-yn-1-ones with isocyanides was concerted, it should be predictable that the model reaction failed to the [4+1+1] cycloaddition product 3a′. To test the role of ligands, we added DPPF or TMEDA to the reaction system under standard conditions (entries 14 and 15). However, there were no obvious changes in yield, implying that ligands might have little effect on this reaction.
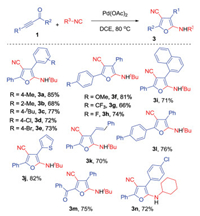
With the optimized conditions (entry 8, Table 1) in hand, we then explored the generality of our method. The results were illustrated in Scheme 2. From the results, an array of 2-amino-4-cycano furans 3 was achieved in good yields accordingly. For example, the reaction of 1-phenyl-3-(thiophen-2-yl)prop-2-yn-1-one 1j under standard conditions provided the corresponding product 3j in 82% yield, while the reaction of 1, 5-diphenylpent-4-en-2-yn-1-one 1k gave rise to corresponding furan 3k in a 70% yield.
|
Download:
|
| Scheme 2. Generation of furans 3 through palladium-catalyzed [4 + 1] cyclization of prop-2-yn-1-ones and isocyanides. Reaction conditions: prop-2-yn-1-ones 1 (0.2 mmol), Pd(OAc)2 (10 mol%), isocyanides (3.0 equiv.), DCE (2 mL), 80 ℃, overnight. Isolated yields based on prop-2-yn-1-ones 1. | |
Particularly, substrate with double carbonyl 1, 4-diphenylbut-3-yne-1, 2-dione 1m was an efficient reaction partner, leading to the desired product 3m in 75% yield. Interestingly, the cyclohexyl isocyanide was also compatible for the reaction, with the formation of corresponding furan 3n in 72% yield. It was noteworthy that the reaction of prop-2-yn-1-imine and cyclohexyl isocyanide did not offer pyrroles, as presented in our previous report. To our surprise, the substrates prop-2-yn-1-ones with either alkylalkyne or alkyl carbonyl failed to produce the corresponding furans (data not shown in Scheme 2).
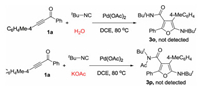
In our previous work, it was found that water and KOAc could serve as efficient partners in reaction of prop-2-yn-1-imine with isocyanides. As such, the three-component reaction of prop-2-yn-1-one, isocyanides, and H2O/KOAc was conducted with a wish to prepare furan derivatives with more morecular diversity and group complexes (Scheme 3). Surprisingly, the corresponding reaction became complex, and no desired products 3o and 3p was obtained as expected.
|
Download:
|
| Scheme 3. Generation of furans 3 through palladium-catalyzed [4 + 1] cyclization of prop-2-yn-1-ones, isocyanides, and H2O/KOAc. Reaction conditions: prop-2-yn-1-ones 1a (0.2 mmol), Pd(OAc)2 (10 mol%), isocyanides (3.0 equiv.), H2O/KOAc (10 equiv.), DCE (2 mL), 80 ℃, overnight. Isolated yields based on prop-2-yn-1-one 1a. | |
Interestingly, the four-component reaction of prop-2-yn-1-ones 1, amines, isocyanides, and H2O worked well under standard conditions. The results were presented in Scheme 3. However, the above four-component reaction did not provide furan derivatives, but various pyrroles. We reasoned that prop-2-yn-1-ones 1 was readily to condensate with amine to in situ generate prop-2-yn-1-imines. Palladium-catalyzed [4 + 1] cycloaddition of prop-2-yn-1-imines with isocyanides was established by our group previously. As such, we exploited the reaction scope on the four-component reaction of prop-2-yn-1-ones 1, amines, isocyanides, and H2O.
As presented in Scheme 4, a series of pyrroles 4 was achieved in good yields accordingly. Pleasingly, prop-2-yn-1-ones 1 herein was expanded to prop-2-yn-1-aldehydes (entries 4a-4m, Scheme 4), prop-2-yn-1-alkylones (entries 4n and 4o, Scheme 4), and alkyl alkyne-connected prop-2-yn-1-ones (entries 4q and 4r, Scheme 4), although amines were limited to aryl amines. The reactions using alkyl amines became complex, probably since 1, 4-addition of alkylamine readily happened to prop-2-yn-1-ones 1.

|
Download:
|
| Scheme 4. Generation of pyrrole 4 through palladium-catalyzed [4 + 1] cyclization of prop-2-yn-1-ones, amines, water, and isocyanides. Reaction conditions: prop-2-yn-1-ones 1 (0.2 mmol), Pd(OAc)2 (10 mol%), isocyanides (3.0 equiv.), H2O (10 equiv.), DCE (2 mL), 80 ℃, overnight. Isolated yields based on prop-2-yn-1-ones 1. | |
In light of the aforementioned results and our previous findings, a plausible mechanism was proposed in Scheme 5. As illustrated in Scheme 5, a [4 + 1] cycloaddition of prop-2-yn-1-ones 1 with isocyanindes to produce furan cation palladium species A. Considering the fact failing to provide [4+1+1] cycloaddition product, it was believed that a [4 + 1] cycloaddition of prop-2-yn-1-ones 1 was concerted. The intermediate A occurred to go through another imidoylation, with the formation of furan cation imidoylpalladium species B [15]. Sequential β-carbon elimination and β-hydride elimination finally released the products 3 [12, 16].
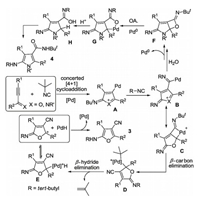
|
Download:
|
| Scheme 5. Plausible mechanism. | |
When treated with water, the intermediate B went through nucleophilic addition of water and reductive elimination to afford bicyclo[3.2.0]hept-4-ene intermediate F. In the presence of palladium, oxidative addition of palladium into intermediate F provided an intermediate G [17], which underwent double protonation to offer the final products 4.
In conclusion, we have developed a palladium-catalyzed concerted [4 + 1] cycloaddition of prop-2-yn-1-ones with double isocyanides. The transformation worked well to produce a series of 2-amino-4-cyanofurans with high efficiency and a broad reaction scope. Treated with aryl amine and H2O, the [4 + 1] cycloaddition of prop-2-yn-1-ones with double isocyanides provided 2-amino-4-amidylpyrroles efficiently.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsFinancial supports from the Natural Science Foundation of Shandong Province (Nos. ZR2019PB004 and ZR2018BB029) and the Natural Science Foundation of China (Nos. 21502069 and 21772067) are gratefully acknowledged.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.04.041.
| [1] |
(a) Q. Wang, D. Wang, M. Wang, et al., Acc. Chem. Res. 51 (2018) 1290-1300; (b) M.M. Heravi, N. Nazari, Curr. Org. Chem. 21 (2017) 1440-1529; (c) M. Giustiniano, A. Basso, V. Mercalli, et al., Chem. Soc. Rev. 46 (2017) 1295-1357; (d) E. Ruijter, R. Scheffelaar, R.V.A. Orru, Angew. Chem. Int. Ed. 50 (2011) 6234-6246; (e) A.V. Lygin, A. Meijere, Angew. Chem. Int. Ed. 49 (2010) 9094-9124. |
| [2] |
(a) B. Song, B. Xu, Chem. Soc. Rev. 46 (2017) 1103-1123; (b) V.P. Boyarskiy, N.A. Bokach, K.V. Luzyanin, et al., Chem. Rev. 115 (2015) 2698-2779; (c) J. Chen, X. Hu, L. Lu, et al., Chem. Rev. 115 (2015) 5301-5365; (d) S. Lang, Chem. Soc. Rev. 42 (2013) 4867-4880; (e) G. Qiu, Q. Ding, J. Wu, Chem. Soc. Rev. 42 (2013) 5257-5269; (f) T. Vlaar, E. Ruijter, B.U. W. Maes, et al., Angew. Chem. Int. Ed. 52 (2013) 7084-7097. |
| [3] |
(a) Z. Gu, S. Ji, Acta Chem. Sin. 76 (2018) 347-356; (b) J. Lei, J. Huang, Q. Zhu, Org. Biomol. Chem. 14 (2016) 2593-2602; (c) H. Wang, B. Xu, Chin. J. Org. Chem. 35 (2015) 588-602; (d) B. Zhang, A. Studer, Chem. Soc. Rev. 44 (2015) 3505-3521. |
| [4] |
(a) S. Otsuka, K. Nogi, H. Yorimitsu, Angew. Chem. Int. Ed. 57 (2018) 6653-6657; (b) J. Chang, B. Liu, Y. Yang, et al., Org. Lett. 18 (2016) 3984-3987; (c) W. Kong, Y. Liu, H. Xu, et al., J. Am. Chem. Soc. 138 (2016) 2146-2149; (d) Z. Gu, J. Li, S. Wang, et al., Chem. Commun. 53 (2017) 11173-11176; (e) H. Wang, P. Mi, W. Zhao, et al., Org. Lett. 19 (2017) 5613-5616; (f) S.R. Shirsath, G.H. Shinde, A.C. Shaikh, et al., J. Org. Chem. 83 (2018) 12305-12314; (g) G. Qiu, C. Sornay, D. Savary, et al., Tetrahedron 74 (2018) 6966-6971. |
| [5] |
(a) M. Lautens, W. Klute, W. Tam, Chem. Rev. 96 (1996) 49-92; (b) I. Ojima, M. Tzamarioudaki, Z. Li, et al., Chem. Rev. 96 (1996) 635-662; (c) H.W. Frühauf, Chem. Rev. 97 (1997) 523-596; (d) T. Kaur, P. Wadhwa, S. Bagchi, et al., Chem. Commun. 52 (2016) 6958-6976; (e) A.V. Gulevich, A.G. Zhdanko, R.V. A. Orru, et al., Chem. Rev. 110 (2010) 5235-5331. |
| [6] |
(a) D. Moderhack, Synthesis (1985) 1083-1096; (b) E. Marchand, G. Morel, S. Sinbandhit, Eur. J. Org. Chem. (1999) 1729-1738; (c) K. Tamao, K. Kobayashi, Y. Ito, J. Am. Chem. Soc. 110 (1988) 1286-1288. |
| [7] |
(a) M. Zhang, S.L. Buchwald, J. Org. Chem. 61 (1996) 4498-4499; (b) X. Wang, X.P. Xu, S.Y. Wang, et al., Org. Lett. 15 (2013) 4246-4249; (c) F. Yi, W. Zhao, Z. Wang, et al., Org. Lett. 21 (2019) 3158-3161; (d) M. Motaghi, H. Khosravi, S. Balalaie, et al., Org. Biomol. Chem. 17 (2019) 275-282; (e) J. George, S.Y. Kim, K. Oh, Org. Lett. 20 (2018) 7192-7196; (f) Z. Hu, J. Dong, Z. Li, et al., Org. Lett. 20 (2018) 6750-6754; (g) Y. He, Y.C. Wang, K. Hu, et al., J. Org. Chem. 81 (2016) 11813-11818; (h) J. Huang, F. Li, L. Cui, et al., Chem. Commun. 56 (2020) 4555-4558. |
| [8] |
(a) N. Chatani, M. Oshita, M. Tobisu, et al., J. Am. Chem. Soc. 125 (2003) 7812-7813; (b) M. Oshita, K. Yamashita, M. Tobisu, et al., J. Am. Chem. Soc. 127 (2005) 761-766. |
| [9] |
P. Fontaine, G. Masson, J. Zhu, Org. Lett. 11 (2009) 1555-1558. DOI:10.1021/ol9001619 |
| [10] |
(a) F. Li, P. Hu, M. Sun, et al., Chem. Commun. 54 (2018) 6412-6415; (b) S. Su, J. Hu, Y. Cui, et al., Chem. Commun. 55 (2019) 12243-12246. |
| [11] |
(a) I. Favler, D. Pla, M. Gomez, Chem. Rev. 120 (2020) 1146-1183; (b) J.K. Liu, J.F. Gong, M.P. Song, Org. Biomol. Chem. 17 (2019) 6069-6098; (c) J. Wang, G. Dong, Chem. Rev. 119 (2019) 7478-7528. |
| [12] |
D. Chen, Y. Shan, J. Li, et al., Org. Lett. 21 (2019) 4044-4048. DOI:10.1021/acs.orglett.9b01220 |
| [13] |
(a) Y.C. Wang, R.X. Wang, G. Qiu, et al., Org. Chem. Front. 6 (2019) 2471-2479; (b) Y.H. Wang, G. Qiu, H. Zhou, et al., Tetrahedron 75 (2019) 3850-3855; (c) Y. Zheng, M. Liu, G. Qiu, et al., Tetrahedron 75 (2019) 1663-1668; (d) G. Qiu, Z. Chen, W. Xie, H. Zhou, Eur. J. Org. Chem. (2019) 4327-4333; (e) K. Huang, J. Li, G. Qiu, et al., RSC Adv. 9 (2019) 33460-33464; (f) Y.H. Wang, B. Ouyang, G. Qiu, et al., Org. Biomol. Chem. 17 (2019) 4335-4341; (g) R. Liu, M. Li, W. Xie, et al., J. Org. Chem. 84 (2019) 11763-11773; (h) Y.C. Wang, J.B. Liu, H. Zhou, et al., J. Org. Chem. 85 (2020) 1906-1914; (i) L. Zhang, L. Ma, H. Zhou, et al., Org. Lett. 20 (2018) 2407-2411. |
| [14] |
A.K. Mahida, S.B. Kale, U. Das, Eur. J. Org. Chem (2017) 6427-6433. |
| [15] |
(a) W.K. Yuan, Y.F. Liu, Z. Lan, et al., Org. Lett. 20 (2018) 7158-7162; (b) X. Sun, W. Wang, Y. Li, et al., Org. Lett. 18 (2016) 4638-4641; (c) C. Masdeu, E. Gomez, N.A. O. Williams, et al., Angew. Chem. Int. Ed. 46 (2007) 3043-3046; (d) Q. Gao, P. Zhou, F. Liu, et al., Chem. Commun. 51 (2015) 9519-9522; (e) W. Hu, J. Li, Y. Xu, et al., Org. Lett. 19 (2017) 678-681; (f) W. Hu, M. Li, G. Jiang, et al., Org. Lett. 20 (2018) 3500-3503; (g) G.C. Sendi, T. -Y. Lu, G.K. Dhandabani, et al., Org. Lett. 19 (2017) 1172-1175; (h) H. Sun, S. Tang, D. Li, et al., Org. Biomol. Chem. 16 (2018) 3893-3896. |
| [16] |
(a) Y. Hu, Z. Shen, H. Huang, ACS Catal. 6 (2016) 6785-6789; (b) D. Gauthier, A.T. Lindhardt, E.P. K. Olsen, J. Am. Chem. Soc. 132 (2010) 7998-8009; (c) B.V. Popp, S.S. Stahl, J. Am. Chem. Soc. 129 (2007) 4410-4422; (d) I.D. Hills, G.C. Fu, J. Am. Chem. Soc. 126 (2004) 13178-13179; (e) X. Li, B. Liu, X. Xu, et al., J. Org. Chem. 77 (2012) 8206-8219; (f) X. Li, B. Liu, X. Yu, et al., J. Org. Chem. 77 (2012) 2431-2440; (g) X. Li, B. Liu, P. Yi, et al., J. Org. Chem. 76 (2011) 2345-2349. |
| [17] |
(a) T. Zhou, G. Li, S. Nolan, et al., Org. Lett. 21 (2019) 3304-3309; (b) C.A. Malapit, D.R. Caldwell, N. Sassu, et al., Org. Lett. 19 (2017) 1966-1969. |
 2021, Vol. 32
2021, Vol. 32