b Analysis and Testing Center, Soochow University, Suzhou 215123, China
Oxindole is a kind of heterocyclic skeleton with important pharmacological activity, which is widely found in natural products and drug molecules [1]. Among them, oxindole derivatives bearing 3-alkenylidene framework have been attracted much attention recently because of novel biological activity [2]. For example, this kind of skeleton is widely found in biological inhibitors Ⅰ [3], AMPK activators Ⅱ [4], anti-rheumatic tenidap Ⅲ [5], and anticancer drug Ⅳ (Fig. 1) [6]. In addition, 3-(allylidene)oxindole format Ⅴ (Fig. 1) is excellent precursor in organic synthesis, which can be used to construct spirooxindoles [7]. Considering the importance of 3-alkenylideneoxindoles as chemical entities, extension of reaction types and modification of the core structure are deemed worthy of pursuit [8].
|
Download:
|
| Fig. 1. Examples of functional 3-methylene- or 3-allylidene-oxindole molecules. | |
Initially, the indolones containing 3-alkenylidene were synthesized by intramolecular cyclization [9] or reductive coupling reaction between indirubin and ketones [10]. In recent years, metal-catalyzed coupling cyclization has become a major strategy for the preparation of 3-allylidene substituted 2-indoleones (Scheme 1).
|
Download:
|
| Scheme 1. Previous work for access to 3-(allylidene)oxindoles by noble-metalcatalysis. | |
Takemoto et al. reported series of multi-component reactions for the synthesis of 3-diarylmethylene oxindoles with palladium catalysts (Schemes 1a, b) [11]. Azhagan and Muthusamy prepared the similar molecular skeleton by rhodium-catalysis (Scheme 1c) [12]. However, most of these reactions require the use of noble metal catalysts, complex ligands and relatively high temperature. Therefore, it is of great significance to discover a metal-free, mild and efficient reaction for the preparation of 3-alkenylidene-2-indolinone framework.
As an important building block in organic synthesis, 3-indolyl alcohol has been widely used to generate fused- or spiro-indoline compounds. Recently, You and Jiao et al. [13, 14] reported the transformation of tryptophols into furoindoline compounds with the metal-catalyst and selectfluor, azide, TEMPO, etc. (Scheme 2a). A longer side-chain substrate 3-indolyl alcohol would lead to pyranoindoline product in moderate yields, which was well-demonstrated in Deng's work (Scheme 2b). Different from those existed reactions, Vincent [15] realized a new reaction for access to spirooxindoles via a cascade of attack of phosphonyl radical and/or trifluoromethyl radical to the C2-position of the indole nucleus under oxidative conditions followed by the intramolecular trapping of the resulting carbocation before rearomatization (Scheme 2c). Despite this, all the reactions above mentioned terminated by hydroxyl cyclization, resulting in O-heterocycles. No further transformation about O-heterocycles was observed.

|
Download:
|
| Scheme 2. Transformation of indolol. | |
We have been interested in the reaction chemistry of indole for years [16]. Recently, we found that a six-step cascade reaction of 3-(1H-indol-3-yl)-1, 1-diarylpropan-1-ol could be realized via NBS-mediation, which led to readily formation of 3-alkenylidene-2-indolinone compounds. Intramolecular oxygen-migration was observed in the reaction, and no metal catalyst was required. Herein, we report these results.
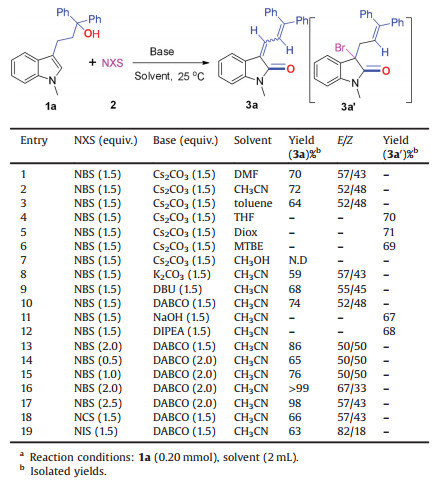
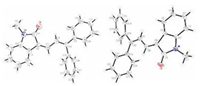
Our research started with the model reaction of 3-(1H-indol-3-yl)-1, 1-diphenylpropan-1-ol (1a) and NBS at room temperature. Firstly, a series of solvents were investigated. The desired products could be obtained in moderate to good yields by variation of the solvents (Table 1, entries 1–3). Among the candidates, CH3CN served as the most suitable solvent (Table 1, entry 2), affording the desired product 3a in 72% yield (Z/E = 52/48). In other solvents such as THF, MTBE, 1, 4-dioxane, a large amount of brominated intermediate 3a′ were generated (Table 1, entries 4–6). No desired product was observed when the model reaction performed in methanol (Table 1, entry 7). Then we screened organic and inorganic bases (Table 1, entries 8–12). Of the bases examined, we found that triethylenediamine (DABCO) was the best choice to give product 3a in highest yield of 74% (Z/E = 52/48) (entry 10). As to NBS (Table 1, entries 13–17), we found that when two equivalent of NBS and DABCO were introduced into the reaction at the same time, the reaction could be quantitatively converted into the target product with an isolated yield more than 99% (Z/E = 67/33) (Table 1, entry 16), two stereoisomers of product 3a can be separated successfully and the definite structures were determined by X-ray crystallographic analysis (Fig. 2). Finally, we also tested the influence of NIS and NCS on the reaction, and it was found no better results than NBS were presented (Table 1, entries 18 and 19).
|
|
Table 1 Optimization of the reaction conditions.a |
|
Download:
|
| Fig. 2. Single crystal structure of (Z)-3a (left) and (E)-3a (right). | |
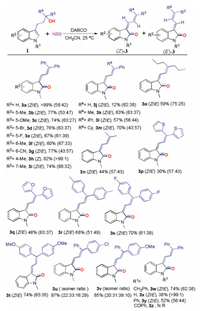
With the optimal conditions in hand, we explored the substrate scope of various 3-(1H-indol-3-yl)propan-1-ol 1 (Scheme 3). Tolerance of functional groups on the benzene ring of indole nucleus was first studied. Electron-donating groups, electron-withdrawing groups or halogens were well tolerated in this reaction (3b-3i), respectively. It should be noted here, when methyl group linked at the position of C5, C6 and C7 on the benzene ring of indole, the reaction gave the products with isomer ratio Z/E from 53:47 to 68:32. However, when methyl group linked at C4 position, only (Z)-3h could be obtained in 82% yield, no E-isomer was observed in the reaction, which might be resulted from the steric effect.
|
Download:
|
| Scheme 3. Substrate scope for reaction of NBS with derivatives of 1. Reaction conditions: 1 (0.5 mmol), NBS (1 mmol), DABCO (1 mmol), CHCN (2 mL), 2 h. | |
Subsequently, we replaced one of the two benzene rings connected to the α-C of hydroxy group with hydrogen or an alkyl group. It was found that when one of the benzene rings was changed to hydrogen, the reaction was greatly hampered, and the target product could only be obtained in 12% yield (3j). In the case of alkyl groups, the reactions could also occur smoothly, and the target products were obtained in moderate to good yields (3j-3m). To our delight, when both benzene rings were replaced by alkyl groups or aromatic heterocycles, the reactions worked well and gave the desired products in moderate yields (3n–3q). Substituents on the benzene rings have trivial effect on the reactions, the selected substrates could also afford products 3r-3t in good yields. Different substituents on the benzene rings led to good yields of products, albeit with 4 configurational isomers (3u and 3v). Beside methyl group, benzyl and phenyl groups were also found to be good substituents on the nitrogen of the indole nucleus, which afforded the target products in 74% and 52% yields (3w and 3y), respectively. If there was no substituent on the nitrogen, the yield of product was further decreased to 38%. Moreover, electron-withdrawing substituent such as benzoyl group resulted in no reaction, which suggested that the electron-rich substrates were preferred to give the desired products.
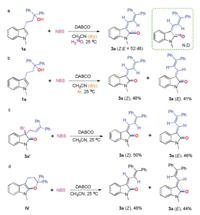
In order to gain insight into the reaction mechanism, several control experiments were conducted (Scheme 4). The experiments with 18O labelled H2O (Scheme 4a) indicate that the oxygen in the product does not come from water. Besides, 3a could be obtained in 89% (Z/E = 54/46) yield when the reaction was performed in dry CH3CN under argon atmosphere (Scheme 4b), which indicate that the oxygen in the product does not come from air. These results meant the oxygen in 3a was from the hydroxy group of 1a.
|
Download:
|
| Scheme 4. Control experiments. | |
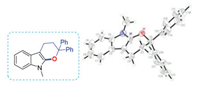
Next, 3a′ was examined as a potential intermediate under the standard conditions, it was found that the products 3a could be obtained in 96% (Z/E = 52/48) total yield (Scheme 4c). Moreover, we were fortunately to get another important intermediate 7 under a lower reaction temperature. The definite structure of 7 was determined by X-ray crystallographic analysis (Fig. 3). Again, we detected 7 under the standard conditions (Scheme 4d), 3a was isolated in 92% yield (Z/E = 48/44). These results indicated that both 3a′ and 7 were intermediates.
|
Download:
|
| Fig. 3. Single crystal structure of 7. | |
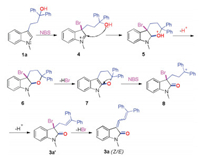
Thus, a plausible mechanism was proposed (Scheme 5). First, the electrophilic attack of bromine bearing positive charge on C3 position of indole generates an intermediate 4 [17]. Then, intramolecular nucleophilic addition takes place to give the intermediate 5. Accompanying with the elimination of the proton as well as HBr in the presence of DABCO, intermediate 7 was thus formed [18]. After further attack by Br+ [19], carbonyl group and carbocation produced with the (Ph2)C—O bond cleavage. With elimination of β-H, the first C=C bond formed. Under this circumstance, the second molecular HBr was abstracted by DABCO, which led to the formation of final products 3a.
|
Download:
|
| Scheme 5. Plausible reaction mechanism. | |
In summary, we have developed an efficient strategy for the construction of 3-(diarylallylidene)oxindoles. With no need of metal catalyst, NBS mediated a six-step cascade conversion of 3-(1H-indol-3-yl)-1, 1-diarylpropan-1-ol into target product, and in which intramolecular oxygen-migration involved. Beside these characteristics, the protocol also has advantages of mild condition, high efficiency, good atom-economy and broad substrate scope. It paved a way for the further study on the application of 3-allylideneindoleones.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe gratefully acknowledge the National Natural Science Foundation of China (Nos. 21772138 and 21672157), PAPD, the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX19_1899), the Project of Scientific and Technologic Infrastructute of Suzhou (No. SZS201708), and State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials for financial support. We thank Can Liu in this group for reproducing the results of 3a, 3u and 3a′.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.070.
| [1] |
(a) C. Marti, E.M. Carreira, Eur. J. Org. Chem. 68 (2003) 2209-2215; (b) A.B. Dounay, L.E. Overman, Chem. Rev. 103 (2003) 2945-2963; (c) C.V. Galliford, K.A. Scheidt, Angew. Chem. Int. Ed. 119 (2007) 8902-8912; (d) V. Chris, Galliford, K.A. Scheidt, Angew. Chem. Int. Ed. 46 (2007) 8748-8758; (e) K.K. Wang, W. Du, J. Zhu, Y.C. Chen, Chin. Chem. Lett. 28 (2017) 512-516. |
| [2] |
(a) L. Sun, C. Liang, S. Shirazian, et al., J. Med. Chem. 46 (2003) 1116-1119; (b) L. Sun, N. Tran, C. Liang, et al., J. Med. Chem. 43 (2000) 2655-2663; (c) S. Lin, Z.Q. Yang, B.H.B. Kwok, et al., J. Am. Chem. Soc. 126 (2004) 6347-6355. |
| [3] |
(a) L. Sun, N. Tran, F. Tang, et al., J. Med. Chem. 41 (1998) 2588-2603; (b) V.R. Solomon, C. Hu, H. Lee, Bioorg. Med. Chem. 17 (2009) 7585-7592. |
| [4] |
L.F. Yu, Y.Y. Li, M.B. Su, et al., ACS Med. Chem. Lett. 4 (2013) 475-480. DOI:10.1021/ml400028q |
| [5] |
R.P. Robinson, L.A. Reiter, W.E. Barth, et al., J. Med. Chem. 39 (1996) 10-18. DOI:10.1021/jm950575k |
| [6] |
A. Pal, A. Ganguly, A. Ghosh, M. Yousuf, B. Rathore, R. Banerjee, S. Adhikari, ChemMedChem 9 (2014) 727-732. DOI:10.1002/cmdc.201400003 |
| [7] |
K.H. Kim, H.R. Moon, J. Lee, J.N. Kima, Adv. Synth. Catal. 357 (2015) 701-708. DOI:10.1002/adsc.201400965 |
| [8] |
Y. Yu, K.J. Shin, J.H. Seo, J. Org. Chem. 82 (2017) 1864-1871. DOI:10.1021/acs.joc.6b02909 |
| [9] |
R.R. Goehring, Y.P. Sachdeva, J.S. Pisipati, M.C. Sleevi, J.F. Wolfe, J. Am. Chem. Soc. 107 (1985) 435-443. DOI:10.1021/ja00288a027 |
| [10] |
(a) N.K. Sasaki, T. Sakurai, Tetrahedron 70 (2014) 9668-9675; (b) L. Chen, Z.J. Wu, L. Peng, et al., Chin. J. Chem. 32 (2014) 844-852; (c) M.A. Loreto, A. Migliorini, P.A. Tardella, A. Gambacorta, Eur. J. Org. Chem. 14 (2007) 2365-2371. |
| [11] |
(a) R. Yanada, S. Obika, T. Inokuma, et al., J. Org. Chem. 70 (2005) 6972-6975; (b) T. Miura, T. Toyoshima, Y. Takahashi, M. Murakami, Org. Lett. 10 (2008) 4887-4889; (c) W.S. Cheung, R.J. Patch, M.R. Player, J. Org. Chem. 70 (2005) 3741-3744. |
| [12] |
S. Muthusamy, D. Azhagan, Tetrahedron Lett. 52 (2011) 6732-6735. DOI:10.1016/j.tetlet.2011.10.004 |
| [13] |
(a) L. Han, C. Liu, W. Zhang, X.X. Shi, S.L. You, Chem. Commun. 50 (2014) 1231-1233; (b) C. Liu, W. Zhang, L.X. Dai, S.L. You, Org. Lett. 14 (2012) 4525-4527; (c) Y.Z. Cheng, Q.R. Zhao, X. Zhang, S.L. You, Angew. Chem. Int. Ed. 58 (2019) 18069-18074. |
| [14] |
(a) O. Lozano, G. Blessley, T.M. Campo, et al., Angew. Chem. Int. Ed. 50 (2011) 8105-8109; (b) H. Yin, T. Wang, N. Jiao, Org. Lett. 16 (2014) 2302-2305; (c) J.J. Guo, S.Y. Chen, J.Y. Liu, et al., Eur. J. Org. Chem. 32 (2017) 4773-4777; (d) X. Deng, K.J. Liang, X.G. Tong, et al., Org. Lett. 16 (2014) 3276-3279; (e) P.P. Zhang, W.S. Sun, G.F. Li, L. Hong, R. Wang, Chem. Commun. 51 (2015) 12293-12296; (f) X.F. Chen, J.B. Fan, G.Y. Zeng, et al., J. Org. Chem. 83 (2018) 8322-8330. |
| [15] |
(a) D. Ryzhakov, M. Jarret, R. Guillot, C. Kouklovsky, G. Vincent, Org. Lett. 19 (2017) 6336-6339; (b) D. Ryzhakov, M. Jarret, J.P. Baltaze, et al., Org. Lett. 21 (2019) 4986-4990; (c) H.F. Wang, D. Liu, H.Y. Chen, J. Li, D.Z. Wang, Tetrahedron 71 (2015) 7073-7076; (d) J. Li, M. Liu, Q. Li, H. Tian, Y.A. Shi, Org. Biomol. Chem. 12 (2014) 9769-9772. |
| [16] |
(a) Y. Zi, Z.J. Cai, S.Y. Wang, S.J. Ji, Org. Lett. 16 (2014) 3094-3097; (b) Z.J. Cai, S.Y. Wang, S.J. Ji, Org. Lett. 15 (2013) 5226-5229; (c) X. Wang, S.Y. Wang, S.J. Ji, Org. Lett. 15 (2013) 1954-1957; (d) W.B. Cao, X.P. Xu, S.J. Ji, Org. Biomol. Chem. 15 (2017) 1651-1654; (e) X.Q. Chu, Y. Zi, H. Meng, X.P. Xu, S.J. Ji, Org. Biomol. Chem. 12 (2014) 4243-4251; (f) W.J. Hao, S.Y. Wang, S.J. Ji, ACS Catal. 3 (2013) 2501-2504; (g) W.B. Cao, X.Q. Chu, Y. Zhou, et al., Chem. Commun. 53 (2017) 6601-6604; (h) M.M. Xu, W.B. Cao, R. Ding, et al., Org. Lett. 21 (2019) 6217-6220; (i) L.L. Zhang, W.B. Cao, X.P. Xu, S.J. Ji, Org. Chem. Front. 6 (2019) 1787-1795; (j) Y. Zhou, X.P. Xu, S.J. Ji, Org. Lett. 21 (2019) 2039-2042. |
| [17] |
(a) P.P. Huang, X.J. Peng, D. Hu, et al., Org. Biomol. Chem. 15 (2017) 9622-9629; (b) Y.R. Li, L.Y. Zhang, H.Y. Yuan, F.S. Liang, J.P. Zhang, Synlett 26 (2015) 116-122. |
| [18] |
(a) Y. Jiang, S.W. Yu, Y. Yang, et al., Org. Biomol. Chem. 16 (2018) 9003-9010; (b) R.B. Ding, Y. Li, C. Tao, B. Cheng, H.B. Zhai, Org. Lett. 17 (2015) 3994-3997. |
| [19] |
Z. Wang, Z.Z. Yu, Y. Yao, et al., Chin. Chem. Lett. 30 (2019) 1541-1544. DOI:10.1016/j.cclet.2019.07.001 |
 2021, Vol. 32
2021, Vol. 32