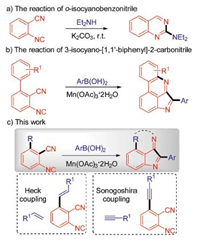
Cyano group is one of the most powerful group in chemical synthesis because it can participate in many reactions and transform into various functional groups, such as amino, amido, and carboxyl [1]. Very recently, the radical reactions involving cyano group have attracted increasing attention for their efficient and diversity applications. These reactions not only led to the carbonyl group [2], but also construct the N-heterocylic compounds [3]. Notably, the radical-mediated cyano group migration strategies which afford the new cyano-containing compounds have been well studied in the past few years [4]. On the other hand, isocyanides, which are isomer with cyano groups, are highly reactive groups and can participate in transition-metal-catalyzed reaction [5] and radical reaction [6]. Especially, the reactions utilizing o-functionalized arylisocyanides to construct the N-heterocylic compounds via radical cascade cyclization strategy in one step have received increasing attention of researchers. There are many o-functionalized arylisocyanides as radical acceptors, such as 2-isocyanobiaryls [7], 2-alkenyl arylisocyanides [8], 2-alkynyl arylisocyanides [9], 1-azido-2-isocyanoarenes [10], o-diisocyanoarenes [11], 2-isocyanothioanisoles [12].
It is of particular interest to develop the reactions of the arylisocyanides which modified by cyano group in the ortho position to afford the N-heterocylic compounds. However, only two relevant works have been reported in the literature. In 1999, Ito and his collaborators reported the reaction of o-isocyanobenzonitile to give the 2-diethylaminoquinazoline (only one example, Scheme 1a) [13], In addition, our group developed the novel method to construct the pyrrolopridin derivatives in this year (Scheme 1b). As our ongoing interest in the reaction of o-isocyanobenzonitile, we designed and synthesized 2-isocyano-6-alkenylbenzonitrile and 2-isocyano-6-alkynylbenzonitrile, respectively. Herein, we demonstrate a radical cascade reaction of 2-isocyano-6-alkenyl(alkynyl)benzonitrile to construct pyrroloisoquinoline derivatives (Scheme 1c).
|
Download:
|
| Scheme 1. The reaction of isocyanides. | |
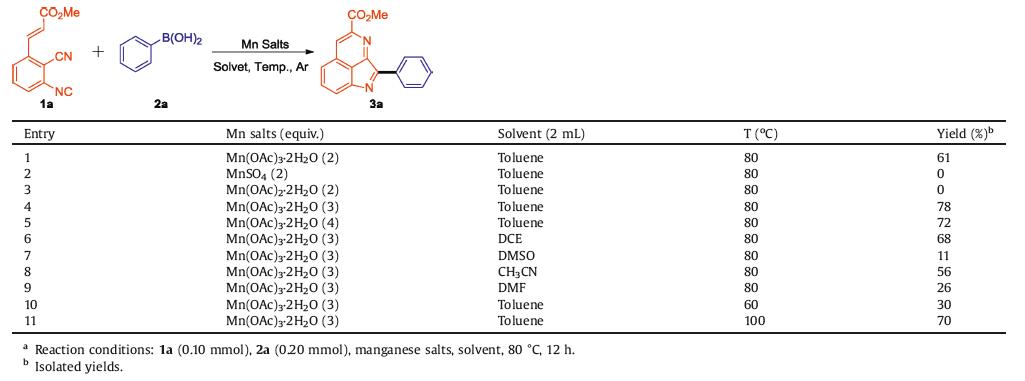
We started our investigations with the reaction of methyl (E)-3-(2-cyano-3-isocyanophenyl)acrylate (1a) and phenylboronic acid (2a) in the presence of 2.0 equiv. of Mn(OAc)3·2H2O in toluene to optimize the reaction. To our delight, the reaction proceeded smoothly to form the target product methyl 2-phenylpyrrolo[2,3,4-ij]isoquinoline-4-carboxylate (3a) in 61% yield after 12 h (Table 1, entry 1). Alternative manganese salts such as MnSO4 and Mn(OAc)2·2H2O, no desired product was detected (Table 1, entries 2 and 3). When increase the loading amount of Mn(OAc)3·2H2O to 3.0 equiv., the reaction proceed smoothly to give 3a in 78% yield (Table 1, entry 4). While, the yield of 3a reduce to 72% in the presence of 4.0 equiv. of Mn(OAc)3·2H2O (Table 1, entry 5). Other solvents including DCE, DMSO, CH3CN and DMF were also investigated under similar reaction conditions. However, all these solvents could not improve the yield of 3a (Table 1, entries 6–9). Notably, either elevating or lowering the temperature resulted in lower yields (Table 1, entries 10 and 11). Therefore, the optimized reaction conditions is following: methyl (E)-3-(2-cyano-3-isocyanophenyl)acrylate (1a) and phenylboronic acid (2a) in the presence of 3.0 equiv. of Mn(OAc)3·2H2O in 2.0 mL toluene at 80 ℃.
|
|
Table 1 Optimization of the reaction conditions a. |
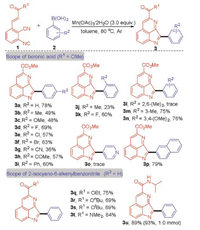
After having established the optimal reaction conditions, we investigated the substrate scope of 2-isocyano-6-alkenylbenzonitriles and arylboronic acids (Scheme 2). The reactions of arylboronic acids which substrates with electron-doating (Me, OMe) or electron-withdrawing groups (F, Cl, Br, CN, COMe) in the para position could proceed smoothly to afford the corresponding products 3b-h in 36%–69% yields. When the methyl group shifted to the ortho or meta position of the aryl ring, the reaction proceeded smoothly to give the desired products 3j and 3m in 23% and 75% yields, respectively. While, when the (2,6-dimethylphenyl)boronic acid 2l was applied to the reaction with 1a, the reaction failed to give the desired product 3l. It is worth noting that when the F atom as an ortho substituent of the arylboronic acid, the yield of the corresponding product 3k increased to 60%. The results indicate that steric effects have a significant effect on the reaction. In addition, the reactions of the (3,4-dimethylphenyl)boronic acid and naphthalen-2-ylboronic acid with 1a could furnish the corresponding products 3n and 3p in 76% and 79% yield, respectively. Unfortunately, when the pyridin-4-ylboronic acid was employed in the reaction with 1a and failed to obtain the desired product 3o under the standard conditions. Then, a series of 2-isocyano-6-alkenylbenzonitrile were synthesized and have been subjected to the reactions with 2a under the optimized conditions. It should be noted that the corresponding products 3q-u were obtained in moderate to excellent yields. To show the potential applications of our method, we tried the reactions of 1f with 2a in 1.0 mmol scale under the standard reaction conditions. To our delight, the reaction proceeded smoothly to afford 3u in 93% yield.
|
Download:
|
| Scheme 2. The reaction of 2-isocyano-6-alkenylbenzonitrile with arylboronic acids. Reaction conditions: 1 (0.1 mmol), 2 (0.2 mmol), Mn(OAc)3·2H2O (3.0 equiv.), toluene (2 mL), 80 ℃, 12 h, argon atmosphere. Isolated yield. 3 u was synthesized in 1.0 mmol scale by 1f (1.0 mmol), 2a (2.0 mmol), Mn(OAc)3·2H2O (3.0 equiv.), toluene (20 mL), 80 ℃, 12 h, argon atmosphere. | |
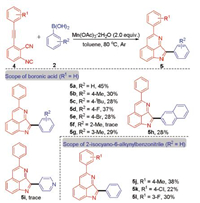
Next, we turned our attention to the reaction of 2-isocyano-6-alkynylbenzonitrile with arylboronic acid (Scheme 3). After extensive optimization, we were able to identify conditions which included 2-isocyano-6-(phenylethynyl)benzonitrile (4a) and phenylboronic acid (2a) in the presence of 2.0 equiv. of Mn(OAc)3·2H2O in toluene at 80 ℃ that delivered the desired product 5a in 45% isolated yield. To further evaluate the scope of this method, differently substituted arylboronic acids were examined. It is worth mentioning that several functional groups, including electron-rich (Me, tBu) or halogen groups (F, Br) of the arylboronic acid as the substrate the corresponding products 5b, 5c, 5d, 5e, 5g were obtained in 28%–37% yields. It is notable that 5f could not be observed when o-tolylboronic acid reacted with 4a due to the steric effect. Additionally, the desired product 5h could be obtained in 28 % yield when using naphthalen-2-ylboronic acid as the substrate. Unfortunately, the reaction of pyridin-4-ylboronic acid with 4a failed to give the corresponding product 5i. We also explored the scope of 2-isocyano-6-alkynylbenzonitrile. Different groups on the aryl ring including methyl (5j), chloro (5k), and fluoro (5l) were also tolerated under the reaction conditions.
|
Download:
|
| Scheme 3. The reaction of 2-isocyano-6-alkynylbenzonitrile with arylboronic acids. Reaction conditions: 4 (0.2 mmol), 2 (0.4 mmol), Mn(OAc)3·2H2O (2.0 equiv.), toluene (6 mL), 80 ℃, 12 h, argon atmosphere. Isolated yield. | |
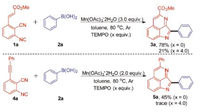
To gain insight into the reaction mechanism, a series of radical capture experiments have been carried out (Scheme 4). When 4.0 equiv. of 2,2,6,6-tetramethylpiperidinyloxy (TEMPO) was employed in the reaction system under the optimized conditions, we could isolated the desired product in 21% yield. The above results which suggest a radical process is probably involved in the reaction of 2-isocyano-6-alkenylbenzonitrile and arylboronic acid. After that, a similar result was observed when 4.0 equiv. of TEMPO was applied to the reaction of 4a and 2a.
|
Download:
|
| Scheme 4. Control experiment. | |
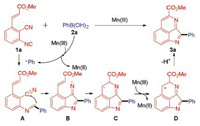
On the basis of the results above and related literatures [3, 14], we proposed a plausible reaction mechanism (Scheme 5). First, the Mn(III) promotes the phenylboronic acid to give the phenyl radical. Next, the radical intermediate A is generated by the reaction of methyl (E)-3-(2-cyano-3-isocyanophenyl)acrylate (1a) and the phenyl radical. Subsequently, the radical intermediate A adds to the cyano group to furnish the corresponding N-centered radical B. The N-centered radical B undergoes intramolecular cyclization with the C—C double bond to afford the radical intermediate C. Then, the intermediate D is afforded via single-electron oxidation of C by Mn(III). Finally, the corresponding product 3a is formed by hydrogen abstraction from the intermediate D.
|
Download:
|
| Scheme 5. Plausible mechanism. | |
In summary, we have developed a radical cascade reaction of 2-isocyano-6-alkenyl(alkynyl)benzonitriles with arylboronic acid in the presence of Mn(III). This reaction provides a novel strategy for the construction of pyrroloisoquinoline derivatives in one step via the formation of two C—C bonds and one C—N bond, which has high step and atomic economy.
Declaration of competing interestThe authors declare no completing financial interest.
AcknowledgmentsWe gratefully acknowledge the National Natural Science Foundation of China (Nos. 21971174, 21772137, 21542015 and 21672157), PAPD, the Project of Scientific and Technologic Infrastructure of Suzhou (No. SZS201708), the Major Basic Research Project of the Natural Science Foundation of the Jiangsu Higher Education Institutions (No. 16KJA150002), Soochow University for financial support.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.023.
| [1] |
(a) M.X. Wang, Acc. Chem. Res. 48 (2015) 602-611; (b) P. Anbarasan, T. Schareina, M. Beller, Chem. Soc. Rev. 40 (2011) 5049-5067. |
| [2] |
(a) S.S. Wang, H. Fu, Y. Shen, M. Sun, Y.M. Li, J. Org. Chem. 81 (2016) 2920-2929; (b) X.T. Zhu, Q. Zhao, F. Liu, et al., Chem. Commun. 53 (2017) 6828-6831; (c) J. Yu, H.X. Sheng, S.W. Wang, et al., Chem. Commun. 55 (2019) 4578-4581. |
| [3] |
(a) J. Zheng, Y. Zhang, D. Wang, S. Cui, Org. Lett. 18 (2016) 1768-1771; (b) X. Li, X. Fang, S. Zhuang, P. Liu, P. Sun, Org. Lett. 19 (2017) 3580-3583; (c) L.J. Wu, Y. Yang, R.J. Song, et al., Chem. Commun. 54 (2018) 1367-1370; (d) X.H. Shan, H.X. Zheng, B. Yang, et al., Nat. Commun. 10 (2019) 908. |
| [4] |
(a) Z. Wu, R. Ren, C. Zhu, Angew. Chem. Int. Ed. 55 (2016) 10821-10824; (b) N. Wang, L. Li, Z.L. Li, et al., Org. Lett. 18 (2016) 6026-6029; (c) Y. Chen, J. Du, Z. Zuo, Chem 6 (2020) 266-279. |
| [5] |
(a) B. Song, B. Xu, Chem. Soc. Rev. 46 (2017) 1103-1123; (b) P. Xu, F. Wang, T.Q. Wei, et al., Org. Lett. 19 (2017) 4484; (c) Y. Fang, J.M. Yang, R. Zhang, S.Y. Wang, S.J. Ji, Org. Chem. Front. 6 (2019) 3383-3386; (d) P. Xu, Y.M. Zhu, X.J. Li, et al., Adv. Synth. Catal. 361 (2019) 4909-4913; (e) G.S. Chen, S.J. Chen, J. Luo, et al., Angew. Chem. Int. Ed. 59 (2020) 614-621. |
| [6] |
(a) B. Zhang, A. Studer, Chem. Soc. Rev. 44 (2015) 3505-3521; (b) J.J. Cao, T.H. Zhu, S.Y. Wang, et al., Chem. Commun. 50 (2014) 6439-6442; (c) J.J. Cao, X. Wang, S.Y. Wang, S.J. Ji, Chem. Commun. 50 (2014) 12892-12895; (d) Y. Fang, C. Liu, F. Wang, et al., Org. Chem. Front. 6 (2019) 660-663. |
| [7] |
(a) M. Tobisu, K. Koh, T. Furukawa, N. Chatani, Angew. Chem. Int. Ed. 51 (2012) 11363-11366; (b) Y. Li, T. Miao, P. Li, L. Wang, Org. Lett. 20 (2018) 1735-1739; (c) A. Guo, J.B. Han, X.Y. Tang, Org. Lett. 20 (2018) 2351-2355; (d) J. Wei, D. Gu, S. Wang, et al., Org. Chem. Front. 5 (2018) 2568-2572; (e) S. Liu, F. Fan, N. Wang, et al., Adv. Synth. Catal. 361 (2019) 3086-3093; (f) S. Liu, W. Pan, S. Wu, et al., Green Chem. 21 (2019) 2905-2910. |
| [8] |
(a) B. Zhang, A. Studer, Org. Lett. 16 (2014) 1216-1219; (b) C.J. Evoniuk, M. Ly, I.V. Alabugin, Chem. Commun. 51 (2015) 12831-12834. |
| [9] |
T. Mitamura, K. Iwata, A. Ogawa, Org. Lett. 11 (2009) 3422-3424. DOI:10.1021/ol901267h |
| [10] |
(a) D. Li, T. Mao, J. Huang, Q. Zhu, Org. Lett. 19 (2017) 3223-3226; (b) D. Li, J. Lei, Org. Biomol. Chem. 17 (2019) 9666-9671. |
| [11] |
(a) D. Leifert, A. Studer, Angew. Chem. Int. Ed. 55 (2016) 11660-11663; (b) X. Sun, W. Wang, Y. Li, J. Ma, S. Yu, Org. Lett. 18 (2016) 4638-4641; (c) Y. Liu, X.L. Chen, F.L. Zeng, et al., J. Org. Chem. 83 (2018) 11727-11735; (d) Y. Fang, C. Liu, W. Rao, S.Y. Wang, S.J. Ji, Org. Lett. 21 (2019) 7687-7691. |
| [12] |
(a) W.C. Yang, K. Wei, X. Sun, J. Zhu, L. Wu, Org. Lett. 20 (2018) 3144-3147; (b) Y. Yuan, W. Dong, X. Gao, X. Xie, Z. Zhang, Org. Lett. 21 (2019) 469-472; (c) K. Luo, W.C. Yang, K. Wei, et al., Org. Lett. 21 (2019) 7851-7856; (d) Y. Liu, X.L. Chen, K. Sun, et al., Org. Lett. 21 (2019) 4019-4024. |
| [13] |
M. Suginome, T. Fukuda, Y. Ito, Org. Lett. 1 (1999) 1977-1979. DOI:10.1021/ol991133w |
| [14] |
P. Xu, Y.M. Zhu, F. Wang, S.Y. Wang, S.J. Ji, Org. Lett. 21 (2019) 683-686. |
 2021, Vol. 32
2021, Vol. 32