b Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
Organosulfur heterocycles are important compounds due to their unique physical properties and versatile biological activity. Thioflavones and benzothiophenes are two class of representative organosulfur heterocycles, and have been widely used as pharmaceuticals, functional materials, and synthetic intermediates [1-7]. Studies have shown that thioflavones are easy to pass through the cell membrane of fungi, changing the ultrastructure of fungal cells, thereby exhibit antibacterial [8] and antiviral activities [9]. So the development of efficient synthetic methods for construction of thioflavone and benzothiophene skeletons has retained the interest of organic researchers along decades of historical development of chemistry [10, 11]. For instance, Schneller's group reported the synthesis of thioflavones by reacting thiophenols and keto esters in 1975 [12]. In 2014, Seijiro's group developed a nickel-catalyzed decarbonylative cyclo-addition reaction of thioisatins and alkynes to form thioflavones [13, 14]. In 2009, Takimiya's group reported a one-pot procedure for the synthesis of benzothiophene derivatives from readily available o-halo-ethynylbenzene precursors [15].
Nowadays, radical cascade reactions have become one of the most efficient synthetic strategy for the construction of organosulfur heterocycles. Thereinto, thioanisole derivatives have been widely used in the radical cyclization process with the release of a methyl group for the construction of thioflavone and benzothiophene skeletons. Most recently, Song and co-workers reported a radical-promoted cyclization of methylthiolated alkynones with diverse radical precursors, and allows an efficient synthesis of a variety of phosphoryl-, sulfenyl-, EtOOCCF2- and acyl-containing thioflavone derivatives under mild conditions (Scheme 1) [16]. Methylthiolated alkynones can also be transferred into 3-aryl [17] and 3-phosphorylated [18] thioflavones by visible-light induced radical reactions with arenediazonium salts and phosphine oxides, respectively. On the other hand, 2-alkynylthioanisoles as versatile building blocks have been widely used in the synthesis of 3-substituted benzothiophenes [19-28].
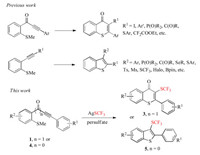
|
Download:
|
| Scheme 1. Syntheses of 3-substituted thioflavones and benzothiophenes. | |
For example, Wu and co-workers disclosed a radical relay strategy for the preparation of 3-(methylsulfonyl)benzothiophenes through a reaction of 2-alkynylthioanisoles with sodium metabisulfite (Scheme 1) in the presence of a photocatalyst under visible light irradiation [29]. Gao and co-workers developed a direct synthetic method for 3-phosphinoylbenzothiophenes through an Ag-mediated radical addition−cyclization of 2-alkynylthioanisoles with secondary phosphine oxides [20].
At the meantime, the special properties of trifluoromethylthio group have led to the widespread use of trifluoromethylthio-containing compounds in many fields, particularly in pharmaceutical and pesticide chemistry [30]. The development of efficient methods for introducing trifluoromethylthio groups into target molecules have attracted much attentions in the field of organic chemistry [31-33]. To date, through the efforts of chemists, direct trifluoromethylthiolation to construct C-SCF3 has achieved rapid development [34-36]. According to the source of trifluoromethylthio group, the direct trifluoromethylthiolation is classified into nucleophilic, electrophilic and free radical pathways [37-54]. In 2014, Wu and co-workers synthesized several 3-trifluoromethylthiobenzothiophenes through the reactions of trifluoromethanesulfanylamide with 2-alkynylthioanisoles promoted by BiCl3 [52]. As part of our ongoing interest in development of synthetic methods for fluorinated compounds, we envisioned that 3-trifluoromethylthio substituted thioflavones and benzothiophenes can be constructed by a radical addition of trifluoromethylthio radical (CF3S·) generated from AgSCF3 to methylthiolated alkynones and 2-alkynylthioanisoles, respectively, followed by an intramolecular radical cyclization reaction (Scheme 1).
Initially, tert-butyl hydroperoxide (TBHP) or benzoyl peroxide (BPO) was chosen as the oxidant, and the reaction of 1-(2-(methylthio)phenyl)-3-phenylprop-2-yn-1-one (1a) with AgSCF3 (2) was conducted in the presence of AgNO3 in CH3CN. But no desired 3-CF3S substituted thioflavone 3a was detected (Table 1, entries 1 and 2). When K2S2O8 was chosen as an oxidant, 3a was formed in 60% yield in the presence of AgNO3 (Table 1, entry 3). However, the yield of 3a was slightly increased in the absence of AgNO3, which indicated that extra transition metal catalyst was not necessary for this reaction (Table 1, entry 4).
|
|
Table 1 Optimization of the reaction conditions.a |
Other persulfate as an oxidant was then screened (Table 1, entries 5 and 6), and the results indicated that (NH4)2S2O8 was the best choice affording the desired product 3a in 75% yield (Table 1, entry 6). The amount of (NH4)2S2O8 was then adjusted, and the results revealed that 1.5 equiv. of oxidant was optimal (Table 1, entries 7 and 8). The yield of product 3a was decreased when the reaction temperature was increased to 90 ℃ or reduced to 70 ℃ (Table 1, entries 9 and 10). Subsequently, different solvents were investigated, and no better yield was observed when the reaction was carried out in different solvents other than CH3CN (Table 1, entries 11–13).
With the optimized reaction conditions in hand (Table 1, entry 6), the efficiency and generality of this reaction was explored, and the results were presented in Scheme 2. The R1 group on the benzene ring of alkynones was first investigated and most of the functional groups were tolerated under the optimized conditions. With electron-donating substituents at the R1 position, such as methyl, methoxy, tert-butyl and phenyl groups, products 3b-3f were obtained in yields of 55%–67%. Halogen atoms such as fluorine, chlorine, and bromine have little influences under the optimized reaction conditions to afford the corresponding products 3g-3k in moderate yields. Electron-withdrawing substituents, such as cyano, trifluoromethyl and nitro groups, were also compatible with the reaction, affording the desired products 3l-3o in yields of 60%–75%. Notably, formyl group as an electron-withdrawing substituent suppressed the radical cyclization significantly due to its high reactivity to radicals [55], the desired product 3p was obtained only in yield of 30%. Substituent such as bromine group at the R2 position were also amenable for this reaction, affording 3q in 65% yield. Additionally, the thiophenyl group could be tolerated as well under the reaction conditions, and the corresponding products 3r and 3s were obtained in 61% and 57% yields, respectively. The structure of thioflavone 3i was further unambiguously established by X-ray diffraction studies. The supplementary crystallographic data can be obtained free of charge from The Cambridge Cryatallographic Data Center (CCDC No. 1968769).
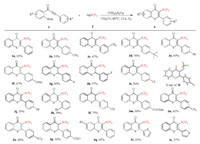
|
Download:
|
| Scheme 2. Synthesis of 3-CF3S substituted thioflavones. Reaction conditions: 1 (0.5 mmol), 2 (1.0 mmol), (NH4)2S2O8 (0.75 mmol), CH3CN (5 mL), 80 ℃, N2, 12 h. Isolated yields. | |
With the above investigation in hand, we assumed that the 2-alkynylthioanisoles would also be suitable for this transformation to afford 3-CF3S substituted benzothiophenes. Thus, the exploration of the reaction of 2-alkynylthioanisoles 4 and AgSCF3 (2) was conducted. The optimal reaction conditions were slightly adjusted with K2S2O8 instead of (NH4)2S2O8 as the oxidant and CH3CN/H2O as a mixed solvent (Supporting information). As shown in Scheme 3, the reactions of 2-alkynylthioanisoles with an electron-donating group at R3 position proceeded smoothly to provide the corresponding products 5a-5e in moderate to good yields. Several sensitive functional groups such as fluoro, chloro, bromo, trifluoromethyl, ester, nitro, cyano, and formyl groups were all compatible. For example, the formyl-containing product 5m was produced in 47% yield. Additionally, methyl substituent at the meta-position of the right benzene ring also presented good reactivity, and the corresponding product 5n was obtained in 80% yield. Then, different substituents on the benzene ring of 2-methylthio-arylalkyne were studied. To our delight, the electronic effects of substituents including methyl, methoxy, trifluoromethoxy, chloro and bromo have no significant influence on the yields of the products, and the expected benzothiophenes 5o-5u were generated in 50%–86% yields. Furthermore, the thiophenyl group could be tolerated as well under the reaction conditions, and the corresponding products 5v and 5w were generated in 65% and 67% yield, respectively. Compared to Wu's protocol reported in 2014 [52], the CF3S source was different and wider substrate scope was investigated in this methodology.
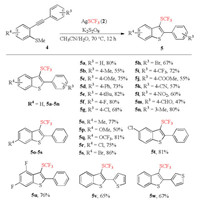
|
Download:
|
| Scheme 3. Synthesis of 3-CF3S substituted benzothiophenes. Reaction conditions: 4 (0.5 mmol), 2 (1.0 mmol), K2S2O8 (0.75 mmol), CH3CN/H2O (v/v = 3:3,6 mL), 70 ℃, 12 h. Isolated yields. | |
With a radical process hypothesized, control experiments were carried out to gain the detailed reaction mechanism. As demonstrated in Scheme 4, the formation of 3a or 5a was completely suppressed after adding 2,2,6,6-tetramethylpiperidine oxide (TEMPO) as a free radical scavenger, and a large amount of starting material 1a or 4a was recovered from the reaction system (Schemes 4a and b). This result indicated that a radical process might be involved in this reaction. In order to verify whether CF3S radical participated in the reaction, F3CSSCF3 was prepared from AgSCF3 and then reacted with 1a. The formation of product 3a was detected in a yield of 60%, which confirmed that CF3S radical was participated in the reaction (Scheme 4c).
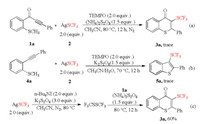
|
Download:
|
| Scheme 4. Control experiments. | |
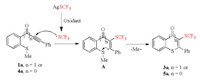
On the basis of these observations and previous reports [21, 27, 28, 56-61], a plausible mechanism for the cascade trifluoromethylthiolation cyclization reaction was described as shown in Scheme 5. Initially, AgSCF3 reacts with oxidant ((NH4)2S2O8 or K2S2O8) to form the CF3S radical. Then, the triple bond in 1a or 4a is attacked by CF3S radical to afford a vinyl radical intermediate A, which follows 6-exo-trig or 5-exo-trig cyclization with the SMe moiety to give the desired product 3a or 5a along with the release of a methyl radical.
|
Download:
|
| Scheme 5. Proposed mechanism. | |
In summary, we have developed an efficient method for synthesis of 3-trifluoromethylthioflavones through a radical addition and cyclization of methylthio substituted arylalkynyl ketones with AgSCF3. Besides, a facile and general route to 3-trifluoromethylthiobenzothiophenes via a reaction of 2-alkynylthioanisoles and AgSCF3 was described. These protocols featured simple operation, mild conditions, good functional group tolerance and high yields.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentWe thank the National Natural Science Foundation of China (Nos. 21991123, 21677094) for financial support.
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.02.040.
| [1] |
J. Dong, Q. Zhang, Q. Meng, et al., Mini Rev. Med. Chem. 18 (2018) 1714-1732. DOI:10.2174/1389557518666180515145633 |
| [2] |
S. Gotoda, N. Takahashi, H. Nakagawa, M. Murakami, et al., Pestic. Sci. 52 (1998) 309-320. DOI:10.1002/(SICI)1096-9063(199804)52:4<309::AID-PS727>3.0.CO;2-J |
| [3] |
D. Dhanak, R.M. Keenen, G. Burton, et al., Bioorg. Med. Chem. Lett. 8 (1998) 3677-3682. DOI:10.1016/S0960-894X(98)00666-0 |
| [4] |
H.K. Wang, K.F. Bastow, L.M. Cosentino, et al., J. Med. Chem. 39 (1996) 1975-1980. DOI:10.1021/jm960008c |
| [5] |
L. Bettinetti, K. Schlotter, H. Huebner, et al., J. Med. Chem. 45 (2002) 4594-4597. DOI:10.1021/jm025558r |
| [6] |
M. Pieroni, E. Azzali, N. Basilico, et al., J. Med. Chem. 60 (2017) 1959-1970. DOI:10.1021/acs.jmedchem.6b01685 |
| [7] |
T. Zhang, D.Q. Xu, X.C. Wang, et al., Chin. Chem. Lett. 27 (2016) 441-446. DOI:10.1016/j.cclet.2015.12.028 |
| [8] |
H. Nakazumi, T. Ueyama, T. Kitao, J. Heterocycl. Chem. 22 (1985) 5497-5501. |
| [9] |
N. Hoettecke, S. Rotzoll, U. Albrecht, et al., Bioorg. Med. Chem. 16 (2008) 10319-10325. DOI:10.1016/j.bmc.2008.10.043 |
| [10] |
S. Sangeetha, G. Sekar, Org. Lett. 21 (2019) 75-79. DOI:10.1021/acs.orglett.8b03508 |
| [11] |
F. Zhu, X.F. Wu, J. Org. Chem. 83 (2018) 13612-13617. DOI:10.1021/acs.joc.8b02294 |
| [12] |
S.W. Schneller, Adv. Heterocycl. Chem. 18 (1975) 59-97. |
| [13] |
I. Tasuku, K. Takuya, M. Seijiro, Org. Lett. 16 (2014) 5660-5662. DOI:10.1021/ol5026102 |
| [14] |
H.Y. Kim, E. Song, K. Oh, Org. Lett. 19 (2017) 312-315. DOI:10.1021/acs.orglett.6b03348 |
| [15] |
T. Kashiki, S. Shinamura, M. Kohara, et al., Org. Lett. 11 (2009) 2473-2475. DOI:10.1021/ol900809w |
| [16] |
J. Xu, F. Zhang, S.F. Zhang, et al., Org. Lett. 21 (2019) 1112-1115. DOI:10.1021/acs.orglett.9b00023 |
| [17] |
B. Alcaide, P. Almendros, E. Busto, et al., Adv. Synth. Catal. 359 (2017) 2640-2652. DOI:10.1002/adsc.201700427 |
| [18] |
X.C. Liu, X.L. Chen, Y. Liu, et al., ChemSusChem 13 (2020) 298-303. DOI:10.1002/cssc.201902817 |
| [19] |
C. An, C.Y. Li, X.B. Huang, et al., Org. Lett. 21 (2019) 6710-6714. DOI:10.1021/acs.orglett.9b02315 |
| [20] |
T. Cai, J. Liu, H. Zhang, et al., Org. Lett. 21 (2019) 4605-4608. DOI:10.1021/acs.orglett.9b01510 |
| [21] |
W. Liu, Y.Q. Hu, X.Y. Hong, et al., Chem. Commun. 54 (2018) 14148-14151. DOI:10.1039/C8CC07735E |
| [22] |
Q. Glenadel, E. Ismalaj, T. Billard, Org. Lett. 20 (2018) 56-59. DOI:10.1021/acs.orglett.7b03338 |
| [23] |
A.J. Warner, A. Churn, J.S. McGough, et al., Angew. Chem. Int. Ed. 56 (2017) 354-358. DOI:10.1002/anie.201610014 |
| [24] |
T. Yamauchi, F. Shibahara, T. Murai, Tetrahedron Lett. 57 (2016) 2945-2948. DOI:10.1016/j.tetlet.2016.05.033 |
| [25] |
R.C. Larock, D. Yue, Tetrahedron Lett. 42 (2001) 6011-6013. DOI:10.1016/S0040-4039(01)01149-2 |
| [26] |
I. Nakamura, T. Sato, Y. Yamamoto, Angew. Chem. Int. Ed. 45 (2006) 4473-4475. DOI:10.1002/anie.200601178 |
| [27] |
J. Yan, J. Xu, Y. Zhou, et al., Org. Chem. Front. 5 (2018) 1483-1487. DOI:10.1039/C8QO00147B |
| [28] |
J. Xu, X. Yu, J. Yan, et al., Org. Lett. 19 (2017) 6292-6295. DOI:10.1021/acs.orglett.7b02971 |
| [29] |
X.X. Gong, M.J. Wang, S.Q. Ye, J. Wu, Org. Lett. 21 (2019) 1156-1160. DOI:10.1021/acs.orglett.9b00100 |
| [30] |
W.R. Dolbier, J. Fluorine Chem. 126 (2005) 157-163. DOI:10.1016/j.jfluchem.2004.09.033 |
| [31] |
Y. Guo, M.M. Huang, J. Chen, et al., Chin. Chem. Lett. 28 (2017) 719-728. DOI:10.1016/j.cclet.2017.02.006 |
| [32] |
X.H. Xu, M. Kohei, S. Norio, Chem. Rev. 115 (2015) 731-764. DOI:10.1021/cr500193b |
| [33] |
H. Chachignon, C. Dominique, Chin. J. Chem. 34 (2016) 445-454. DOI:10.1002/cjoc.201500890 |
| [34] |
L. Grégory, P. Armen, P. Sergiy, et al., Beilstein J. Org. Chem. 9 (2013) 2476-2536. DOI:10.3762/bjoc.9.287 |
| [35] |
C. Chen, L. Chu, F.L. Qing, J. Am. Chem. Soc. 134 (2012) 12454-12457. DOI:10.1021/ja305801m |
| [36] |
F. Baert, J. Colomb, T. Billard, Angew. Chem. Int. Ed. 51 (2012) 10382-10385. DOI:10.1002/anie.201205156 |
| [37] |
G. Teverovskiy, D.S. Surry, S.L. Buchwald, Angew. Chem. Int. Ed. 50 (2011) 7312-7314. DOI:10.1002/anie.201102543 |
| [38] |
A.L. Barthelemy, E. Magnier, G. Dagousset, Synthesis 50 (2018) 4765-4776. DOI:10.1055/s-0037-1611278 |
| [39] |
T. Liu, G. Qiu, Q. Ding, J. Wu, Tetrahedron 72 (2016) 1472-1476. DOI:10.1016/j.tet.2016.01.053 |
| [40] |
C.P. Zhang, D.A. Vicic, J. Am. Chem. Soc. 134 (2012) 183-185. DOI:10.1021/ja210364r |
| [41] |
Z. Weng, W. He, C. Chen, et al., Angew. Chem. Int. Ed. 52 (2013) 1548-1552. DOI:10.1002/anie.201208432 |
| [42] |
Y.D. Yang, A. Azuma, E. Tokunaga, et al., J. Am. Chem. Soc. 135 (2013) 8782-8785. DOI:10.1021/ja402455f |
| [43] |
L. Zhuo, J. Long, Y.C. Cao, et al., Chin. Chem. Lett. 30 (2019) 714-716. DOI:10.1016/j.cclet.2018.11.013 |
| [44] |
A. Tlili, T. Billard, Angew. Chem. Int. Ed. 52 (2013) 6818-6819. DOI:10.1002/anie.201301438 |
| [45] |
A. Ferry, T. Billard, B.R. Langlois, E. Bacque, Angew. Chem. Int. Ed. 48 (2009) 8551-8555. DOI:10.1002/anie.200903387 |
| [46] |
X. Wang, T. Yang, X. Cheng, Q. Shen, Angew. Chem. Int. Ed. 52 (2013) 12860-12864. DOI:10.1002/anie.201305075 |
| [47] |
S.Y. Lu, W.B. Chen, Q.L. Shen, Chin. Chem. Lett. 30 (2019) 2279-2281. DOI:10.1016/j.cclet.2019.07.060 |
| [48] |
J. Sheng, S. Li, J. Wu, Chem. Commun. 50 (2014) 578-580. DOI:10.1039/C3CC45958F |
| [49] |
Q. Xiao, J. Sheng, Z. Chen, J. Wu, Chem. Commun. 49 (2013) 8647-8649. DOI:10.1039/c3cc44263b |
| [50] |
Q. Xiao, J. Sheng, Q. Ding, J. Wu, Eur. J. Org. Chem. (2014) 217-221. |
| [51] |
R. Pluta, P. Nikolaienko, M. Rueping, Angew. Chem. Int. Ed. 53 (2014) 1650-1653. DOI:10.1002/anie.201307484 |
| [52] |
J. Sheng, C. Fan, J. Wu, Chem. Commun. 50 (2014) 5494-5496. DOI:10.1039/c4cc01904k |
| [53] |
P. Luo, Q. Ding, Y. Ping, J. Hu, Org. Biomol. Chem. 14 (2016) 2924-2929. DOI:10.1039/C6OB00005C |
| [54] |
Y. Li, G. Li, Q. Ding, Eur. J. Org. Chem. (2014) 5017-5022. |
| [55] |
S.Y. Chang, Y.F. Shao, S.F. Chu, et al., Org. Lett. 1 (1999) 945-948. DOI:10.1021/ol990199o |
| [56] |
D.P. Hari, T. Hering, B. Köning, Org. Lett. 14 (2012) 5334-5337. DOI:10.1021/ol302517n |
| [57] |
M.K. Staples, R.L. Grange, J.A. Angus, et al., Org. Biomol. Chem. 9 (2011) 473-479. DOI:10.1039/C0OB00573H |
| [58] |
F.E. McDonald, Burova Jr. S.A., Huffman, Synthesis 7 (2000) 970-974. |
| [59] |
H. Zang, J.G. Sun, X. Dong, et al., Adv. Synth. Catal. 358 (2016) 1746-1752. DOI:10.1002/adsc.201501102 |
| [60] |
W.C. Yang, K. Wei, X. Sun, et al., Org. Lett. 20 (2018) 3144-3147. DOI:10.1021/acs.orglett.8b01278 |
| [61] |
Y.Z. Gao, P.B. Zhang, G. Li, Y.F. Zhao, J. Org. Chem. 83 (2018) 13726-13733. DOI:10.1021/acs.joc.8b02001 |
 2021, Vol. 32
2021, Vol. 32 


