b Key Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang 550025, China;
c College of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590, China
Dodine (1-dodecylguanidinium acetate) is a widely used low-toxicity and protective fungicide (Scheme 1). It is used to control a variety of major mold diseases on fruit trees, vegetables, nuts, ornamental plants, and shade trees. Its main principle as a fungicide is that it can easily adsorb on the surface of negatively charged microorganisms, penetrate their membranes, and destroy their cell structure. It thus has a sterilizing effect and eliminates algae [1-3]. In addition, it is used as a disinfectant in industrial water treatment agents, detergents, and seed treatment agents, and to disinfect women's and children's products as well as food packaging [4, 5]. However, its use is also associated with some risks [6, 7]. Structurally, it is a cationic surface-active compound, and it essentially exhibits the toxicological properties characteristic of this class of compounds in single or repeated doses. Its most conspicuous toxic action is a pronounced irritant effect of the solid or concentrated solutions on skin and mucous membranes.

|
Download:
|
| Scheme 1. Structures of Q[10], PBA and dodine | |
Due to the widespread use of dodine, methods for its detection and quantification are required, and indeed some such methods have been published. Dodine shows an ultraviolet (UV) absorption maximum at about 200 nm, and so above a certain concentration it may be detected by liquid chromatography with UV monitoring. Therefore, residual amounts in fruit are currently mainly determined by liquid chromatography-electrospray ionization mass spectrometry (LC/ESI-MS). Kazuhito et al. [8] used LC/ESI-MS to detect dodine in agricultural products. Their method involved extraction with acetonitrile and then re-extraction with ethyl acetate. The extract was cleaned-up on a PSA cartridge column, and finally analyzed by LC/MS. Newsome detected dodine residues in foods by gas chromatography [9]. The procedure involved extraction with methanol, partitioning with chloroform, and derivatization with hexafluoroacetyl acetone. Other similar methods have also been reported [10, 11]. Although the above methods have low detection limits, they tend to be more cumbersome, time-consuming, and, most importantly, require expensive and sophisticated instrumentation. Thus, a new simple and fast method remains highly desirable.
With the rapid development of various novel fluorescent compounds, the field of supramolecular chemistry has benefited from their encapsulation in suitable hosts, such as cyclodextrins [12], calix[n]arenes [13], and pillar[n]arenes [14-20]. Cucurbit[n]urils (Q[n]s) [21, 22], as a class of macrocyclic host molecules, have also been employed to construct fluorescent probes. For example, in 2012, Xing et al. [23] detected paraquat in aqueous solution by using the complex of cucurbit[7]uril and acridine orange. Del Pozo and Hernández also used cucurbit[7]uril as a host to detect carbendazim in oranges in 2010 [24]. Recently, our group has applied a fluorescent probe based on twisted cucurbit[14]uril and thioflavin T for detecting flusilazole [25].
In order to further expand the application of Q[n] in detecting pesticides, we have now designed a water-soluble probe with fluorescence as its key feature. Pyrene is a large conjugated fluorophore, and its fluorescence makes it a suitable guest for our purposes. In order to overcome the problem of low water-solubility of the conjugated arene, a long chain with a diamine structure was appended to afford aminopropyl-1-pyrenebutanamide (PBA, Scheme 1). The synthetic route is outlined in Fig. S1 (Supporting information). Considering the large size of the pyrene moiety of the guest, Q[10] with a large cavity was selected as the host. Consequently, a fluorescent probe was constructed from cucurbit[10]uril and PBA, which is designated as PBA@Q[10]. It was obtained by simple mixing of Q[10] and PBA in aqueous solution in a 1:1 stoichiometry. The PBA@Q[10] probe was then used to detect 20 different pesticides of varying shape and size, namely dinotefuran, thiamethoxam, acetamiprid, pyroquilon, dodine, metalaxyl, pyrimethanil, oxadixyl, carbaryl, ethiofencarb, pymetrozine, azaconazole, tebuconazole, triadimenol A, penconazole, flutriafol, flusilazole, triadimefon, tricyclazole and paraquat (Fig. S5 in Supporting information). On adding tenfold excesses of the respective pesticides to the PBA@Q[10] probe, only dodine elicited a strong fluorescence enhancement effect, while the other pesticides reduced the fluorescence to different extents. The results clearly demonstrate that our probe can be used for the selective detection of dodine. Our findings not only expand the application of host-guest cucurbit[n]uril chemistry in everyday life, but also indicate a new and effective means of detecting dodine.
The ability of PBA to form a complex with Q[10] was monitored by 1H NMR and fluorescence emission. Host-guest complexation was firstly assessed by 1H NMR titration, whereby Q[10] was incrementally added to a 50 mmol/L solution of PBA in D2O (Fig. 1). Addition of 1.0 equiv. of Q[10] resulted in upfield shifts of the signals of protons Ha-f, p of the guest. That is to say, the proton signals of the pyrene group of PBA showed obvious upfield shifts. Meanwhile, the signals of protons Hb-f also showed gradual upfield shifts, moving from δ 1.44, 2.77, 3.23, 2.02 and 2.20 to δ 2.11-1.34. The signal of proton Ha was upfield shifted from δ 2.62 to δ 2.27. These dramatic upfield shifts for the proton signals of PBA upon addition of Q[10], compared with those of the free guest, suggest that in the complex PBA@Q[10], the PBA is included in the cavity of Q[10]. Given the large cavity associated with Q[10], the Q[10] can also shuttle on the guest PBA in a state of dynamic equilibrium [26-28].

|
Download:
|
| Fig. 1. (above) Possible interaction between PBA and Q[10]; (below) 1H NMR titration spectra of PBA with the addition of different molar equivalents of Q[10]: (ⅰ) free guest PBA, (ⅱ) 0.50, (ⅲ) 0.75, (ⅳ) 1.0. | |
The interaction between Q[10] and PBA was further verified by fluorescence emission spectroscopy. As shown in Fig. 2, PBA displays an emission peak at 390 nm in aqueous solution at an excitation wavelength of 332 nm. Incremental addition of Q[10] caused a decrease in the fluorescence intensity at 390 nm and an increase in that at 488 nm, and the fluorescence reached a minimum at an NQ[10]/NPBA ratio of 1:1. The association constant (Ka) of PBA@Q[10] was calculated as 1.06 × 103 L/mol according to the fluorescence spectrum in water (Fig. S7 in Supporting information). A Job plot (Fig. 2b) further corroborated the stoichiometry of the host-guest inclusion complex. Meanwhile, the luminescent color of the solution was also significantly modified; the purple luminescence of PBA was converted into cyan luminescence from the complex PBA@Q[10] under a 365 nm lamp. Moreover, confocal fluorescence microscopy images (Fig. S8 in Supporting information) showed that the fluorescence of PBA was enhanced in the presence of Q[10], due to the formation of PBA@Q[10] [29].

|
Download:
|
| Fig. 2. (a) Fluorescence spectra of PBA (2 × 10-5 mol/L) with increasing molar equivalents of Q[10] from 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2… to 3.0; (b) Job plot for Q[10] and PBA. | |
As mentioned above, simple mixing of Q[10] and PBA in aqueous solution in a 1:1 stoichiometry furnished the fluorescent probe PBA@Q[10]. Its interesting fluorescence properties prompted us to investigate its applicability for the rapid detection of pesticides. To this end, we carried out a series of fluorescence measurements on the abovementioned 20 pesticides (Fig. S5). The results are shown in Fig. 3. Interestingly, when dodine was added to an aqueous solution of PBA@Q[10], the fluorescence characteristics of the probe showed dramatic changes. Its fluorescence intensity at 390 nm was strongly enhanced, and this was accompanied by fluorescence quenching at 488 nm. However, addition of any of the other 19 pesticides to the same aqueous probe solution did not lead to distinct fluorescence changes at 390 nm, only slight fluorescence quenching at 488 nm. Moreover, with the addition of dodine, the significant increase in fluorescence intensity of the probe could be visually perceived (Fig. 4), with the color changing from cyan to blue under a UV lamp. These observations indicate that the probe PBA@Q[10] permits highly selective recognition of dodine in aqueous solution.

|
Download:
|
| Fig. 3. Fluorescence spectra indicating the selective detection of dodine among 20 pesticides. | |

|
Download:
|
| Fig. 4. (Top) Histogram of the fluorescence intensities of PBA in the presence of 20 different pesticides; (bottom) photographs of vials showing the fluorescence emission changes. | |
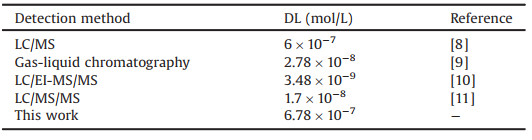
The effect of varying dodine concentrations on the fluorescence intensity of the PBA@Q[10] complex was also investigated. As shown in Fig. 5a, PBA@Q[10] exhibits fluorescence maxima at 390 and 488 nm in the absence of dodine. With the addition of increasing amounts of dodine, the fluorescence intensity at 488 nm is effectively quenched, while that at 390 nm is obviously enhanced.
|
Download:
|
| Fig. 5. (a) Fluorescence spectra of PBA@Q[10] (1 × 10-6 mol/L) with increasing molar equivalents of dodine from 0, 1.0, 2.0…to 50.0; (b) plot of DL. | |
The fluorescence intensity (F) exhibited a good linear relationship with dodine concentration within a certain concentration range. From the plot in Fig. 5b, the detection limit (DL) for dodine was calculated as 6.78 × 10-7 mol/L. This DL value is up to an order of magnitude lower than those for some previously reported detection methods (Table 1). This reiterates that our probe permits highly selective and sensitive recognition of dodine in aqueous solution.
|
|
Table 1 Comparison of detection limits (DL) for dodine by different analytical methods. |
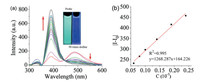
To further verify the mechanism of the fluorescence enhancement of PBA@Q[10], titration 1H NMR spectra was acquired. The interaction between Q[10] and dodine was first examined by 1H NMR spectroscopic analysis. As shown in Fig. S11 (Supporting information), upon the addition of Q[10] to a solution of dodine in D2O, all of the proton signals exhibited distinct upfield shifts compared to those of free dodine, indicating that the whole dodine molecule was encapsulated within the cavity of Q[10]. The association constant (Ka) between Q[10] and dodine was calculated as 8.02 × 104 L/mol by 1H NMR titration (Fig. S13 in Supporting information). In addition, PBA added into the solution of dodine in D2O does not induce any change in the resonances of all the protons for both PBA and dodine, indicating the lack of interaction between PBA and dodine (Fig. S14 in Supporting information).
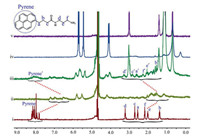
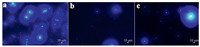
By mixing Q[10] and PBA in a 1:1 stoichiometry in D2O, the 1H NMR spectrum of the PBA@Q[10] inclusion complex was obtained (Fig. 6ⅱ). Upon subsequent addition of dodine to this complex, obvious downfield shifts of all the proton signals attributed to unbound PBA were observed (Fig. 6ⅰ, ⅲ), suggesting that PBA was gradually expelled from the cavity of Q[10]. Although the 1H NMR spectrum still showed the presence of PBA@Q[10], the presence of some free PBA reveals the mode of action. At the same time, the peaks of the protons of dodine show slight upfield shifts (Fig. 6ⅳ, ⅴ and Fig. S15 in Supporting information). All of these observations imply that with the incremental addition of dodine, it gradually displaces PBA from the cavity of Q[10] [25, 30]. Indeed, the Ka of dodine@Q[10] is higher than that of PBA@Q[10], which would explain the significant changes in the fluorescence spectrum of PBA@Q[10] with the gradual addition of dodine. Moreover, the confocal microscopy images showed that the fluorescence of PBA@Q[10] was a significant decreased in addition of dodine, and the fluorescence intensity is close to free PBA (Fig. 7 and Fig. S16 in Supporting information), which supports the proposed mechanism as well.
|
Download:
|
| Fig. 6. Titration 1H NMR spectra of (ⅰ) free PBA, (ⅱ) PBA@Q[10], (ⅲ) PBA@Q[10]-dodine, (ⅳ) dodine@Q[10], and (ⅴ) free dodine. | |
|
Download:
|
| Fig. 7. Confocal microscopy images of (a) 20 μmol/L probe, (b) 20 μmol/L probe-dodine and (c) 20 μmol/L neat PBA. | |
In summary, a new fluorescent probe has been constructed in water based on the host-guest complexation between Q[10], with a large rigid cavity, and PBA, which shows strong fluorescence. The obtained fluorescent probe, PBA@Q[10], has been used to determine dodine among 20 different pesticides, namely dinotefuran, thiamethoxam, acetamiprid, pyroquilon, dodine, metalaxyl, pyrimethanil, oxadixyl, carbaryl, ethiofencarb, pymetrozine, azaconazole, tebuconazole, triadimenol A, penconazole, flutriafol, flusilazole, triadimefon, tricyclazole, and paraquat. The fluorescent probe showed highly selective and sensitive recognition of dodine in aqueous solution. The DL was as low as 6.78 × 10-7 mol/L, similar to those achieved with large precision instruments. Furthermore, the mechanism of the response of the probe towards dodine has been corroborated by 1H NMR spectra: dodine displaces PBA from the cavity of Q[10]. Eventually, the fluorescence intensity of PBA returns to its original level. The present work provides a new approach for extending the application of cucurbit[n]uril-based fluorescent sensors based on host-guest interactions for the detection of other pesticides.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank the National Natural Science Foundation of China (No. 21861011), the Major Program for Creative Research Groups of Guizhou Provincial Education Department (No. 2017-028), the Innovation Program for High-level Talents of Guizhou Province (No. 2016-5657) and the Science and Technology Fund of Guizhou Province (Nos. 2016-1030, 2018-5781) are gratefully acknowledged for financial support.
Appendix A. Supplementary dataSupplementarymaterial related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.02.023.
| [1] |
X.J. Xia, Y.Y. Huang, L. Wang, et al., Pest. Biochem. Phys. 86 (2006) 42-48. DOI:10.1016/j.pestbp.2006.01.005 |
| [2] |
M.L. Gullino, P. Leroux, C.M. Smith, Crop Prot. 19 (2000) 1-11. DOI:10.1016/S0261-2194(99)00095-2 |
| [3] |
A. Paul, S. Nag, K. Sinha, Int. J. Sci. Res. Publ. 3 (2013) 1-7. |
| [4] |
J. Jurewicz, W. Hanke, Int. J. Occup. Med. Environ. Health 19 (2006) 152-169. |
| [5] |
J. Jurewicz, W. Hanke, C. Johansson, et al., Acta Paediatr. Suppl. 95 (2006) 71-80. DOI:10.1080/08035320600886489 |
| [6] |
G.J. Levinskas, L.B. Vidone, J.J. Oâ Grady, et al., Toxicol. Appl. Pharmacol. 3 (1961) 127-142. DOI:10.1016/0041-008X(61)90016-3 |
| [7] |
N. Çördük, N. Akıncı, G. Yücelv, et al., Fresen. Environ. Bull. 24 (2015) 4527-4531. |
| [8] |
M. Aoyagi, K. Niiyama, S. Takatsuki, et al., J. Food Hyg. Soc. Jpn. 50 (2009) 58-63. DOI:10.3358/shokueishi.50.58 |
| [9] |
W.H. Newsome, J. Agricul, Food Chem. 24 (1974) 997-999. |
| [10] |
R. Le Grand, F. Saint-Marcoux, S. Dulaurent, et al., J. AOAC Int. 94 (2011) 300-306. DOI:10.1093/jaoac/94.1.300 |
| [11] |
M. Scordino, L. Sabatino, P. Traulo, et al., Eur. Food Res. Technol. 227 (2008) 1339-1347. DOI:10.1007/s00217-008-0849-3 |
| [12] |
N.L. Pacioni, A.V. Veglia, Anal. Chim. Acta 583 (2007) 63-71. DOI:10.1016/j.aca.2006.10.010 |
| [13] |
X. Zeng, J. Ma, L. Luo, et al., Org. Lett. 17 (2015) 2976-2979. DOI:10.1021/acs.orglett.5b01075 |
| [14] |
P. Wang, X.Z. Yan, F.H. Huang, Chem. Commun. 50 (2014) 5017-5019. DOI:10.1039/c4cc01560f |
| [15] |
Q. Li, H. Zhu, F.H. Huang, J. Am. Chem. Soc. 141 (2019) 13290-13294. DOI:10.1021/jacs.9b05054 |
| [16] |
B. Shi, K. Jie, Y. Zhou, et al., J. Am. Chem. Soc. 138 (2016) 80-83. DOI:10.1021/jacs.5b11676 |
| [17] |
S. Guo, X. Liu, C. Yao, et al., Chem. Commun. 52 (2016) 10751-10754. DOI:10.1039/C6CC05993G |
| [18] |
Y. Cao, Y. Li, X. Hu, et al., Chem. Mater. 27 (2015) 1110-1119. DOI:10.1021/cm504445r |
| [19] |
M. Zuo, W. Qian, T. Li, et al., ACS Appl. Mater. Interfaces 10 (2018) 39214-39221. DOI:10.1021/acsami.8b14110 |
| [20] |
S. Guo, Y. Song, Y. He, et al., Angew. Chem. Int. Ed. 57 (2018) 3163-3167. DOI:10.1002/anie.201800175 |
| [21] |
S. Liu, P.Y. Zavalij, L. Isaacs, J. Am. Chem. Soc. 127 (2005) 16798-16799. DOI:10.1021/ja056287n |
| [22] |
Y. Yu, Y. Li, X. Wang, et al., J. Org. Chem. 82 (2017) 5590-5596. DOI:10.1021/acs.joc.7b00400 |
| [23] |
X. Xing, Y. Zhou, J. Sun, et al., Anal. Lett. 46 (2013) 694-705. DOI:10.1080/00032719.2012.729240 |
| [24] |
M. Del Pozo, L. Hernández, C. Quintana, Talanta 81 (2010) 1542-1546. DOI:10.1016/j.talanta.2010.02.066 |
| [25] |
Y. Fan, R. Gao, Y. Huang, et al., Front. Chem. 7 154. |
| [26] |
W.L. Mock, N.Y. Shih, Org. Chem. 51 (1986) 4440-4446. DOI:10.1021/jo00373a018 |
| [27] |
P.H. Dixit, R.V. Pinjari, S.P. Gejji, J. Phys. Chem. A 114 (2010) 10906-10916. DOI:10.1021/jp107289s |
| [28] |
W.T. Xu, M. Liu, M.C. Escaño, et al., New J. Chem. 43 (2019) 7028-7034. DOI:10.1039/C9NJ01089K |
| [29] |
W.T. Xu, J.L. Kan, B. Yang, et al., Chem. Asian J. 14 (2019) 235-242. DOI:10.1002/asia.201801498 |
| [30] |
S.K. Samanta, J. Quigley, B. Vinciguerra, et al., J. Am. Chem. Soc. 139 (2017) 9066-9074. DOI:10.1021/jacs.7b05154 |
 2021, Vol. 32
2021, Vol. 32