b College of Chemistry and Chemical Engineering, Henan University of Technology, Zhengzhou 450001, China
Self-assembly exists universally in chemistry, materials science and biology. Supramolecular self-assembly based on host-guest chemistry has become an important research topic in recent years due to its wide application in the construction of self-healing materials [1], fluorescence resonance energy transfer (FRET)-based light-harvesting devices [2], molecular machine [3], controlled release [4], photoinduced electron transfer (PET) systems [5] and so on. Since Ogoshi and co-workers first synthesized para-bridged symmetrical pillar[5]arenes in 2008 [6], pillararenes (especially pillar[5]arene and pillar[6]arene) as the fifth generation macrocyclic hosts after crown ether, cucurbituril, cyclodextrin and calixarene are booming. Pillararene has been usually utilized as a host media to construct host-guest complexes and supramolecular polymers [7]. Huang et al. developed a nanoparticle of pillararene-induced near-infrared emission enhancement through host-guest complexation, showing effective PH-responsive ability [8]. Wang et al. developed multiple-stimuli-responsive supramolecular vesicles based with pillar[6]arene and SAINT, achieving controllable release of an anticancer drug, i.e., DOX [9]. Yang's group constructed a [2]biphenyl-extended pillar[6]arene/tetraphenylethylene-based supramolecular polymer, achieving assembly-induced emission enhancement for efficient mercury(Ⅱ) detection and removal [10]. Meanwhile, the group also developed a supramolecular polymer network based on a monofunctionalized leaning tower[6]arene, exhibiting tunable fluorescence characteristic [11].
On the other hand, PET reactions are prominent processes in nature and many areas of science and technology, among which are solar energy conversion, photocatalysis, synthetic organic photo-chemistry, super-resolution imaging, and photodynamic therapy [12]. Supramolecular systems based on PET attracted researchers' extensive attention for easy construction, stimuli-adjustment, conducive to tracking, and so on. Recently, Liu et al. reported a series of PET-based supramolecular architectures mediated by calixarene and cyclodextrin [13]. However, only a few of pillararene-mediated PET systems have been exploited. For instance, Stoddart et al. constructed porphyrinic supramolecular daisy chains incorporating pillar[5]arene and viologen, where PET process from porphyrin to viologen occurred [14]. Electron-rich triarylamine groups usually act as an electron donor, and their derivatives are usually used to construct organic photoelectric materials. Electron-deficient Ru(Ⅱ) bis(terpyridine) derivatives are generally applied in metal-organic catalytic reaction. Despite Yao et al. reported a linear supramolecular polymer comprised of terpyridine monomodified pillar[5]arene with a guest through host-guest complexation and metal (Zn2+) coordination, achieving stimuli-responsive glue–sol transitions [15]. In the present work, a pure metal complex, i.e., Ru(Ⅱ) bis(terpyridine)-modified pillar[5]arenes (TPY-P5(Ru2+)) as host assembles with triazoletriphenylamine amyl cyanide (TTAC) as guest through host-guest interaction to fabricate a PET-based supramolecular system, achieving tunable fluorescence and PET, which is a new research and has not be exploited, to our knowledge.
Compounds TPY-P5(Ru2+) and TTAC were successfully synthesized, and their synthetic routes were shown in Scheme 1. The reaction of substance 1 [16] with 2 [17] obtained terpyridine-modified pillar[5]arene TPY-P5 in 75% yield, and further complexed with Ru(DMSO)4Cl2 to get TPY-P5(Ru2+) in 60% yield. Guest TTAC was prepared from alkyne 3 [18] and azide 4 [19] through "Click" reaction in 73% yield. Compound 2 reacted with n-tetradecyl bromide to obtain intermediate TPY in 93% yield, and further complexed with Ru(DMSO)4Cl2 to prepare reference substance TPY(Ru2+) in 71% yield. Here, TPY was modified with the long alkyl chain (as a solubilizing agent) to increase the solubility of TPY(Ru2+). The chemical structures of all the new compoundsabove were confirmed by 1H NMR, 13C NMR, and HR-MS, which were shown in Figs. S1−S15 (Supporting information).

|
Download:
|
| Scheme 1. The synthetic routes of host TPY-P5(Ru2+), guest TTAC and reference compound TPY(Ru2+). | |
The successful preparation of TPY-P5(Ru2+) and TTAC inspired us to investigate their assembling behavior. It is well known that large binding ability (Ka = 1.2 × 104 L/mol) exists between P5 and the neutral guests bearing two or three short alkyl chains with a triazole site and a cyano site at either end in chloroform [20]. We assumed that TPY-P5(Ru2+) and TTAC might self-assemble to form a 3:2 (host/guest) supramolecular architectures. However, steric hindrance would become seemingly a key influencing factor for whether 3:2 host-guest binding pattern formed. Therefore, it is very necessary for us to examine whether the guest TTAC can complex with three P5s. From NMR titration spectroscopy (Fig. S16 in Supporting information), an apparent upfield shift of the resonance for methylene in the guest (Ha-d) was observed, and their integral areas achieved unchanged with continued addition of 3 equiv. P5, indicating that one TTAC can assemble with three P5s. The complex stability constant (KS) was determined to be 6.52 × 108 M−3 using the 1H NMR single point method [21]. Furthermore, the binding stoichiometry of TPY-P5(Ru2+) with TTAC was verified by a Job plot, where a maximum peak at a molar ratio of 0.6 (Fig. S17 in Supporting information) was observed, revealing a 3:2 host–guest binding stoichiometry. Besides, a direct evidence came from the comparison of 1H NMR spectroscopy. As illuminated in Fig. 1, the similar upfield shift for the resonance of methylene (Ha-d) of the guest was presented, and all the signal peaks was changed to be passivated, implying that the occurrence of the assembling behavior and the formation of a new supramolecular polymer TTAC⊂TPY-P5(Ru2+). The assembling pattern was further confirmed by NOSEY and ROSEY spectra (Figs. S18 and S19 in Supporting information) that the methylenes between cyano group and triazole in TTAC were located in the cavity of the P5 in TPY-P5(Ru2+).

|
Download:
|
| Fig. 1. 1H NMR (600 MHz, v(CD3CN): v(CDCl3) = 1:9) spectra of (a) host TPY-P5(Ru2+), (b) assembly TTAC⊂TPY-P5(Ru2+) and (c) guest TTAC; [TPY-P5(Ru2+)] = 3 × 10−3 mol/L, [TTAC] = 2 × 10-3 mol/L. | |
Moreover, the intuitional proof on topology morphology formed automatically by the TTAC⊂TPY-P5(Ru2+) came from microscopic investigation. As shown in Fig. 2a, many nanoparticles were observed in the TEM image, which further verified by SEM and AFM images (Fig. 2b and Fig. S20 in Supporting information). Moreover, the hydrodynamic diameter of the assembly in CHCl3 was detected as about 36.77 nm and 525.8 nm, indicating the formation of two types of nanoaggregates, i.e., oligomers and polymers in dilute solution. Meanwhile, the aforementioned observations also implied that oligomers formed by the assembly transformed gradually to a large size of polymers in solvent evaporation process (Fig. S21 in Supporting information). Above all, three jaw TTAC and bridged-bis-P5 TPY-P5(Ru2+) can self-organized to form nanoparticles in weak polar solvent. The possible mechanism for nanoparticle formation should be that the supramolecular assembly TTAC⊂TPY-P5(Ru2+) first formed twisted and random reticular nanostructures and then further intertwined to near spherical nanoparticles, as presented in Scheme 2.

|
Download:
|
| Fig. 2. (a) TEM image of assembly TTAC⊂TPY-P5(Ru2+); (b) SEM image of assembly TTAC⊂TPY-P5(Ru2+). | |

|
Download:
|
| Scheme 2. The chemical structures of host TPY-P5(Ru2+), guest TTAC, reference substance TPY(Ru2+) and TTAC⊂TPY-P5(Ru2+) and its schematic illustration of assembling and disassembling pattern. | |
Viewing the structural feature of the host and guest, triphenylamine derivatives donated with lone pair electron usually act as electron donor and bis-terpyridyl ruthenium complexes with electron deficiency can play a role of electron acceptor. We supposed that the resultant supramolecular assembly might achieve PET between the host and guest originated from short enough intermolecular D-A distance. Subsequently, the photophysical properties of the guest, host and assembly were performed. The UV–vis absorption spectra of them were carried out and displayed in Fig. S22 (Supporting information). As we predicted, when 1.5 equiv. TPY-P5(Ru2+) was gradually added to the chloroform solution containing TTAC, the fluorescence of the guest at 390 nm was dramatically quenched by approximately 94%, manifesting the occurrence of "efficient PET" from triphenylamine to bis-terpyridyl ruthenium (2+) complex (Fig. 3a) [22].

|
Download:
|
| Fig. 3. (a) The variation of fluorescent spectra of TTAC with continuous addition of TPY-P5(Ru2+) in chloroform; excitation at 338 nm (slit = 2.5, 2.5). (b) The variation of fluorescent spectra of TTAC with continuous addition of TPY-P5(Ru2+) in DMSO; excitation at 338 nm (sli = 2.5, 1). (c) The variation of fluorescent spectra of TTAC with continuous addition of TPY(Ru2+) in chloroform; excitation at 338 nm (slit = 2.5, 2.5). (d) The photographs of fluorescent quenching. [TTAC] = 1 × 10−5 mol/L. | |
To demonstrate the important role of the PET process played by P5, some control experiments were performed. As illuminated in Fig. 3b, when chloroform solvent was changed to strong polar solvent such as DMSO, ∼56% fluorescent intensity of TTAC at 398 nm was quenched in the presence of 1.5 equiv. TPY-P5(Ru2+), which was much lower than that in chloroform. This result was attributed to weak binding ability of the guest and host in DMSO than in chloroform. As displayed in Fig. S23 (Supporting information), there were many unassembled host and guest in the system. Moreover, more quantitative evidence was provided by NMR titration experiment. From Fig. S24 (Supporting information), the KS of the guest and P5 in DMSO was determined to be 1.53 × 107 M−3, which was greatly lower than that in chloroform (6.52 × 108 M−3). Furthermore, we selected TPY(Ru2+) without P5 as a reference compound to further verify our viewpoint, where the long alkyl chain was introduced to enhance the solubility of the metal-organic complex. Subsequently, fluorescent titration experiment was carried out. As shown in Fig. 3c, fluorescence intensity of TTAC at 390 nm was quenched by only 40% with continued addition of 1.5 equiv. TPY(Ru2+), manifesting that "attenuated PET" occurred. Hence, in this PET system, P5 played a key role in the "efficient PET" process via shortening the intermolecular electron D-A distance through supramolecular complexation. Interestingly, the guest and assembly showed great differences on the maximum fluorescence emission wavelength and intensity in CHCl3 and DMSO. As displayed in Figs. S25 and S26 (Supporting information), the maximum fluorescence manifested bathochromic-shift by 8 nm, when the solvent was changed from CHCl3 to DMSO. Simultaneously, the fluorescence intensity of the guest was enhanced by a factor of 23 in this process. More surprisingly, the fluorescence intensity of the assembly was strongly increased by a factor of 913. These variations were originated from that the guest and assembly formed different stack models in diverse solvent. Subsequently, the fluorescence spectra of different volume ratio of CHCl3/DMSO solution containing the guest and assembly were performed. As displayed in Figs. S27 and S28 (Supporting information), the maximum emission of the guest and assembly both manifested obvious red-shift along the DMSO volume content from 0% to 30%, and the fluorescence intensity exhibited enhanced trend with the increased of the DMSO volume content from 40% to 90%. These experiments both revealed that the guest and assembly both possessed aggregation-induced emission enhancement (AIEE) property. Of an interest is that the supramolecular nanoassembly became a fluorescent switch triggered by solvent conversion. More quantitatively, we selected the guest and assembly in DMSO to investigate the variation of their fluorescence quantum yield (ΦF) to avoid test error because of very weak fluorescence intensity for them in CHCl3. As manifested in Fig. S29 (Supporting information), the ΦF of TTAC was apparently declined from 31.98% to 6.92% with addition of 1.5 equiv. TPY-P5(Ru2+), which further implied the occurrence of PET process.
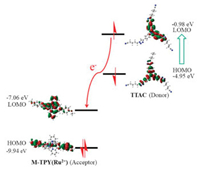
To further demonstrate that the above-mentioned fluorescence quenching was indeed attributed to PET, the relevant optimized structures and HOMO-LOMO orbital energy was performed. Here, we selected 4-methoxytridipyridine (M-TPY(Ru2+)) instead of TPY-P5(Ru2+) with TPY(Ru2+) to calculate their HOMO and LOMO orbital energy. As shown in Table S1 (Supporting information) and Fig. 4, the LOMO energy level (-0.98 eV) of TTAC (electron donor) is greatly higher than M-TPY(Ru2+) as electron acceptor (-7.06 eV), which completely meets the conditions for the occurrence of PET. Therefore, a new PET system was witnessed.
|
Download:
|
| Fig. 4. The proposed photoinduced electron transfer mechanism of M-TPY(Ru2+) and TTAC. | |
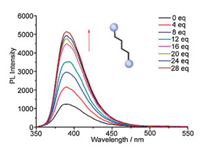
Interestingly, the fluorescent intensity and intermolecular PET process of the resultant assembly were modulated by another competitive guest due to unsubstantial combination of the host and guest [23]. When excess adiponitrile (ADN) was gradually added in the solution containing the supramolecular assembly, the quenching fluorescent intensity of TTAC⊂TPY-P5(Ru2+) at 390 nm got partial recovery, implying that the supramolecular assembly was destroyed to some extent (Fig. 5). To prove the speculation, the NMR contrast experiment was subsequently performed. As illuminated in Fig. S30 (Supporting information), the 1H NMR spectroscopy of free host TPY-P5(Ru2+) and guest TTAC was presented in the presence of excess ADN, manifesting that TTAC was ejected from the cavity of P5 in TPY-P5(Ru2+) by ADN. This showed that the resultant supramolecular assembly TTAC⊂TPY-P5(Ru2+) was completely dissociated and meanwhile, a new ternary supramolecular assembly ADN⊂TPY-P5(Ru2+) was produced, as illuminated in Scheme 2.
|
Download:
|
| Fig. 5. The variation of fluorescent spectrum of TTAC⊂TPY-P5(Ru2+) with continuous addition of ADN; [TTAC] = 1 × 10−5 mol/L, [TPY-P5(Ru2+)] = 1.5 × 10−5 mol/L; Excitation at 338 nm (slit = 5, 2.5). | |
In conclusion, a multi-component metal-organic supramolecular nanoarchitectures TTAC⊂TPY-P5(Ru2+) comprised of TPY-P5(Ru2+) and TTAC had been constructed through host-guest complexation. The assembling pattern and formed topological morphology were demonstrated by NMR, NOSEY, ROSEY, SEM, TEM, AFM and DLS. Interestingly, the supramolecular assembly achieved "efficient PET" from TTAC to TPY-P5(Ru2+), where the host-guest complexation played an important role in shortening the D-A distance. Subsequently, the occurrence of PET process was further proved by HOMO-LOMO orbital energy calculation. Crucially, the fluorescence and PET process of the assembly could be modulated by another competing guest to some extent. The present study provided a versatile strategy to develop smart metal-organic supramolecular materials based with PET for controllable photoelectric transforming.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe thank the National Natural Science Foundation of China (Nos. 21801063, 21672192, 21803059 and 21929101), China Postdoctoral Science Foundation (No. 2018M642767), Ph.D. Foundation of Henan University of Technology, China (No. 2017BS020), the Science and Technology Foundation of Henan Province (No. 192102210039), the Colleges and Universities Key Research Program Foundation of Henan Province (No. 19A150022), Fundation of Henan University of Technology (No. 2018QNJH14), and the Natural Science Foundation of Henan Province (No. 182300410255) for financial support.
Appendix A. Supplementary dataSupplementary material related tothis article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.02.024.
| [1] |
H. Chen, H. Ma, S. Wu, H. Tian, Angew. Chem. Int. Ed. 53 (2014) 14149-14152. DOI:10.1002/anie.201407402 |
| [2] |
J.J. Li, Y. Chen, J. Yu, N. Cheng, Y. Liu, Adv. Mater. 29 (2017) 1701905. DOI:10.1002/adma.201701905 |
| [3] |
S. Ikejiri, Y. Takashima, M. Osaki, H. Yamaguchi, A. Harada, J. Am. Chem. Soc. 140 (2018) 17308-17315. DOI:10.1021/jacs.8b11351 |
| [4] |
D.S. Guo, K. Wang, Y.X. Wang, Y. Liu, J. Am. Chem. Soc. 134 (2012) 10244-10250. DOI:10.1021/ja303280r |
| [5] |
(a) D.S. Guo, Y. Liu, Chem. Soc. Rev. 41 (2012) 5907-5921; (b) D.S. Guo, Y. Liu, Acc. Chem. Res. 47 (2014) 1925-1934. |
| [6] |
T. Ogoshi, S. Kanai, S. Fujinami, T.A. Yamagishi, Y. Nakamoto, J. Am. Chem. Soc. 130 (2008) 5022-5023. DOI:10.1021/ja711260m |
| [7] |
(a) M. Xue, Y. Yang, X. Chi, X. Yan, F. Huang, Chem. Rev. 115 (2015) 7398-7501; (b) G. Yu, K. Jie, F. Huang, Chem. Rev. 115 (2015) 7240-7303; (c) H. Zhang, Z. Liu, Y. Zhao, Chem. Soc. Rev. 47 (2018) 5491-5528; (d) P. Wei, X. Yan, F. Huang, Chem. Soc. Rev. 44 (2015) 815-832; (e) P. Li, Y. Chen, Y. Liu, Chin. Chem. Lett. 30 (2019) 1190-1197; (f) X. Wu, L. Gao, J. Sun, X.Y. Hu, L.Y. Wang, Chin. Chem. Lett. 27 (2016) 1655-1660; (g) S. Sun, J. Shi, Y. Dong, et al., Chin. Chem. Lett. 24 (2013) 987-992; (h) N. Strutt, D. Fairen, J. Iehl, et al., J. Am. Chem. Soc. 134 (2012) 17436-17439; (i) X. Li, Y. Li, J. Wu, et al., J. Mater. Chem. A 8 (2020) 3651-3657; (j) M. Wu, Y.W. Yang, Polym. Chem. 10 (2019) 2980-2985; (k) H. Li, Y. Yang, F. Xu, et al., Chem. Commun. 55 (2019) 271-285; (l) Y. Li, Z. Li, Q. Lin, Y.W. Yang, Nanoscale (2020), doi: http://dx.doi.org/10.1039/C9NR09532B; (m) N. Song, X.Y. Lou, W. Hou, et al., Macromol. Rapid Commun. 39 (2018) 1800593; (n) S. Sun, J. Shi, Y. Dong, et al., Chin. Chem. Lett. 24 (2013) 987-992; (o) X. Wu, L. Gao, J.Z. Sun, X.Y. Hu, L.Y. Wang, Chin. Chem. Lett. 27 (2016) 1655-1660. |
| [8] |
B. Shi, K. Jie, Y. Zhou, et al., J. Am. Chem. Soc. 138 (2016) 80-83. DOI:10.1021/jacs.5b11676 |
| [9] |
Y. Cao, X.Y. Hu, Y. Li, et al., J. Am. Chem. Soc. 136 (2014) 10762-10769. DOI:10.1021/ja505344t |
| [10] |
D. Dai, Z. Li, J. Yang, et al., J. Am. Chem. Soc. 141 (2019) 4756-4763. DOI:10.1021/jacs.9b01546 |
| [11] |
Z. Liu, J. Wu, C. Wang, et al., Chin. Chem. Lett. 30 (2019) 2299-2303. DOI:10.1016/j.cclet.2019.10.023 |
| [12] |
(a) M.K. Brennaman, R.J. Dillon, L. Alibabaei, et al., J. Am. Chem. Soc. 138 (2016) 13085-13102; (b) M.R. Gill, J.A. Thomas, Chem. Soc. Rev. 41 (2012) 3179-3192; (c) H. Huang, B. Yu, P. Zhang, et al., Angew. Chem. Int. Ed. 41 (2015) 14049-14052; (d) R.F. Laine, G.S. K. Schierle, S. van de Linde, C.F. Kaminski, Methods Appl. Fluoresc. 4 (2016) 022004; (e) D.L. Ashford, M.K. Gish, A.K. Vannucci, et al., Chem. Rev. 115 (2015) 13006-13049; (f) N.A. Romero, D.A. Nicewicz, Chem. Rev. 116 (2016) 10075-10166. |
| [13] |
(a) Y.M. Zhang, Y. Chen, Y. Yang, P. Liu, Y. Liu, Chem. Eur. J. 15 (2009) 11333-11340; (b) D.S. Guo, K. Chen, H.Q. Zhang, Y. Liu, Chem. Asian J. 4 (2009) 436-445; (c) K.R. Wang, D.S. Guo, B.P. Jiang, Y. Liu, Chem. Commun. 48 (2012) 3644-3646; (d) Y. Liu, K.R. Wang, D.S. Guo, B.P. Jiang, Adv. Funct. Mater. 19 (2009) 2230-2235; (e) B.P. Jiang, D.S. Guo, Y. Liu, J. Org. Chem. 76 (2011) 6101-6107. |
| [14] |
M. Fathalla, N.L. Strutt, S. Sampath, et al., Chem. Commun. 51 (2015) 10455-10458. DOI:10.1039/C5CC03717D |
| [15] |
B. Shi, K. Jie, Y. Zhou, D. Xia, Y. Yao, Chem. Commun. 51 (2015) 4503-4506. DOI:10.1039/C5CC00535C |
| [16] |
J.F. Ni, X.Y. Hu, J.L. Jiang, L.Y. Wang, Chem. Commun. 50 (2014) 1317-1319. DOI:10.1039/C3CC47823H |
| [17] |
L. Ding, Y.M. Zhang, X. Teng, Y. Liu, J. Org. Chem. 76 (2011) 1910-1913. DOI:10.1021/jo102311m |
| [18] |
P.Z. Li, X.J. Wang, S.Y. Tan, et al., Angew. Chem. Int. Ed. 54 (2015) 12748-12752. DOI:10.1002/anie.201504346 |
| [19] |
L.B. Meng, D. Li, S. Xiong, et al., Chem. Commun. 51 (2015) 4643-4646. DOI:10.1039/C5CC00398A |
| [20] |
(a) C. Li, K. Han, J. Li, et al., Chem. Eur. J. 19 (2013) 11892-11897; (b) X. Wang, H. Deng, J. Li, et al., Macromol. Rapid Commun. 34 (2013) 1856. |
| [21] |
L. Ma, S. Wang, C. Li, et al., Chem. Commun. 54 (2018) 2405-2408. DOI:10.1039/C8CC00213D |
| [22] |
H. Zhu, J. Fan, J. Wang, H. Mu, X. Peng, J. Am. Chem. Soc. 136 (2014) 12820-12823. DOI:10.1021/ja505988g |
| [23] |
Z.Y. Li, Y.Y. Zhang, C.W. Zhang, et al., J. Am. Chem. Soc. 136 (2014) 8577-8589. DOI:10.1021/ja413047r |
 2021, Vol. 32
2021, Vol. 32 

