b Institute of Environmental Science, Department of Chemistry, Shanxi University, Taiyuan 030006, China;
c Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital, Sichuan University, Chengdu 610041, China
Among recent research in the field of host-guest chemistry, pillar[n]arenes are undoubtedly one of the most thoroughly studied molecular hosts [1-7], due to their unique macrocyclic framework, versatile complexation properties, and ready chemical modification [8-14]. So far, the studies on pillar[n]arene derivatives mainly focused on their host-guest complexation properties and the functions derived therefrom [15-23]. By virtue of their relatively strong binding ability, pillar[n]arenes have demonstrated promising in applications such as stimuli-responsive materials [24, 25], molecular recognition [26, 27], energy transfer [28-30] and molecular absorption and separation [31-33]. On the other hand, pyrene is the most representative polyaromatic compound that can emit excimer fluorescence [34-40]. The excimer of pyrene derivatives typically forms at high concentrations. Reinforced intermolecular association between fluorophores [35, 41-44] or covalently linking two or more pyrenes were effective ways to improve excimer formation [45-48]. The photophysical properties of pyrene could be well manipulated by the supramolecular assembly, and the pyrene excimer-based supramolecular systems have been applied for sensing, optical materials and devices [49-51]. We have demonstrated that supramolecular hosts could significantly switch photochemical properties of photo-substrates [52-58] and energy transfer efficiency could be drastically improved by the complexation of pillar[5]arene [28]. In the present communication, we report a novel possible application of pillar[5]arene on the basis of not its host-guest complexation properties but rather the template effect of well-orderly arranged composing units. Thus, a pyrene-tiaraed pillar[5] arene derivative demonstrated unique photophysical and photochemical properties so as to innovate a novel way of photo-writing.
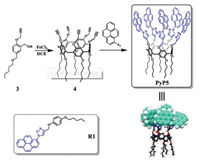
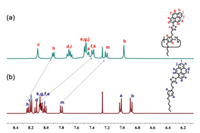
The C5 symmetric PyP5 (Scheme 1) was synthesized followed a strategy for the synthesis of the rim-differentiated tiara-pillar[5] arene, developed independently by Ma [59] and Sue [60] groups, respectively. Thus, the asymmetrically substituted 2, 5-dialkox-ybenzyl alcohol 3 was synthesized by reacting 2, 5-dihydroxyben-zaldehyde stepwise with 1-bromopentane and then 3-bromoprop-1-yne under alkaline conditions. The rim-differentiated tiara-pillar[5]arene 4 was synthesized by catalyzing 3 with FeCl3 [60]. By introducing pyrenes through the azide-alkyne cycloaddition "click" reaction, the pyrene-tiaraed PyP5, in which five pyrenes were orderly arranged on the same rim of the pillar[5]arene (Scheme 1), was obtained in good yield (41.0%). The introduction of the alkyl chains on the rim of the other side allowed PyP5 to be well soluble in many organic solvents. The aromatic proton signals of PyP5 in the 1H NMR spectrum are relatively simple and gave only one set of the protons of pyrenyl and two kinds of hydroquinone aromatic proton signals (Fig. S10 in Supporting information), confirming the C5 symmetric structure. Comparing to the 1H NMR signals of R1, the aromatic proton signals of PyP5 are much more upfield shifted (Fig. 1), which could be ascribed to the mutual shielding effect among pyrene moieties [60, 61].
|
Download:
|
| Scheme 1. Synthetic route of the pyrene-tiaraed PyP5. | |
|
Download:
|
| Fig. 1. Partial 1H NMR spectra (400 MHz, CDCl3, 298 K) of (a) PyP5 and (b) R1. | |
The UV–vis spectrum of PyP5 showed intensive peaks at 290 nm and 365 nm, corresponding to the absorption of the pillar[5]arene moiety and the 1La band of the pyrene units, respectively. The 1La absorption band of PyP5 was apparently bathochromic shifted in comparison of that of R1, suggesting the π-π stacking interaction among pyrene moieties. We were interested to find that PyP5 showed a light blue emission upon excitation with a 365-nm UV lamp. On the contrary, only purple emission peaked at the UV region of 330 nm was observed for R1 in the dichloromethane solution at a concentration of 10.0 μmol/L, indicating that monomer fluorescence dominates at this concentration. Excimer fluorescence appeared when increasing the concentration of R1 to 0.50 mmol/L. Further increasing the concentration of R1 led to a decrease of the monomer fluorescence and an increase of the excimer fluorescence, exhibiting a typical concentration-promoted excimer formation. The broad excimer fluorescence peaked at 476 nm became dominant only at the concentration higher than 5.0 mmol/L (Fig. S17 in supporting information).
On the contrary, PyP5 showed exclusively the excimer fluorescence at 2.0 μmol/L (Fig. 2a), and only tiny monomer fluorescence peaked at 379 nm could be observed. The excimer fluorescence of PyP5 exhibited a peak at 473 nm (Fig. S18 in Supporting information), which is slightly hyperchromic shifted relative to that of R1 (476 nm), possibly due to the relatively rigid framework of pillar[5]arene which prevents pyrenes to stack perfectly [35, 62]. The excimer fluorescence of PyP5 could not be well fitted by one-component exponential decay but rather a two-components model to give lifetimes of 29.9 ns (74%) and 46.1 ns (26%), respectively. This suggests that at least two kinds of excimer fluorescence was formed, for which different types of stacking due to the restriction of the pillar[5]arene framework should be responsible. On the other hand, the lifetime of monomeric fluorescence of R1 measured at 379 nm was 46.1 ns, while the excimer fluorescence at 476 nm gave a lifetime of 53.3 ns (Figs. S17 and S20 in supporting information). Interestingly, monomer fluorescence was negligible even diluting the concentration of PyP5 to down to 1 ×10-8 mol/L (Fig. 2b), indicating that the excimer fluorescence is concentration-independent. These results demonstrate that the excimer fluorescence is mainly an intramolecular process by virtue of the close orientation of pyrene units. The quantum yield of the PyP5 was 26.6%, which is higher than that of R1 (17.1%) (Table 1).

|
Download:
|
| Fig. 2. (a) UV–vis spectra and the normalized fluorescence emission spectrum of PyP5 (Red, 2.0 μmol/L), R1 (black, 10.0 μmol/L) in DCM at room temperature (λex = 330 nm). (b) Fluorescence spectra of PyP5 at different concentration measured in DCM at room temperature (λex = 330 nm). The inset shows the normalized emission spectra. | |
|
|
Table 1 Photophysical properties of PyP5 and R1.a |
The fluorescence spectra of PyP5 were measured in different solvents, including dichloromethane, DMF, DMSO, THF, 1, 4-dioxane, toluene, chloroform and n-hexane, and overwhelming excimer fluorescence was observed irrespective of the solvent used (Fig. S21 in Supporting information). These results further confirmed that the congested and orderly arrangement of the five pyrene moieties guaranteed the extremely high efficiency in the formation of the excimer, which is not disturbed by the solvation. The fluorescence excitation spectra of a 1.0 μmol/L PyP5 solution monitored at 379 and 399 nm were practically superimposable with each other, which significantly deviated to longer wavelengths (~15 nm) when monitored at 473 nm (Fig. S22 in Supporting information), indicating the formation of the intermolecular pyrene static excimer due to the interaction at the ground state [35, 41, 48].
Interestingly, the fluorescence of PyP5 exhibited significant change upon irradiation at 365 nm with a UV lamp (Fig. 3). As can be seen from Fig. 3a, the exposure of PyP5 to a 365nm-LED lamp led to a decrease of excimer emission at 473 nm, accompanied by an increase of monomer emission at 382 nm, which caused a visibly noticeable color change with the naked eye. Since pillar[5]arene framework has no absorption at 365 nm, the photoreaction should occur on the pyrene or the conjugated 1, 2, 3-triazole moieties. Pyrene is known to undergo photochemical conversion upon photolysis in different solvents [63, 64]. On the other hand, 1, 2, 3-triazole may be photodegraded upon photolysis [65, 66]. Indeed, photolysis of pyrene or R1 with the 365 nm-LED lamp led to clear chemical conversion, which could be traced by the fluorescence spectral change (Fig. S23a in Supporting information). The UV–vis spectra of R1 showed a decrease at 344 nm and a slight increase at 375 nm upon irradiation, which is consistent with the UV–vis spectral changes of PyP5 under the same conditions (Fig. 3b). Similarly, the absorbance of pyrene gradually decreased at 335 nm but increased at 365 nm with the irradiation time (Fig. S24b in Supporting information), accompanied by the decrease of the fluorescence intensity (Fig. S23b in supporting information). In contrast to the decrease of the monomer fluorescence upon photolysis of pyrene or R1, the photolysis of PyP5 showed a decrease in the visible emission of excimer fluorescence and an increase of the monomer emission in the UV region, furthermore, one of the photoproducts was separated and characterized to be pyrene (Fig. S26 in supporting information). These results suggested that the fluorescence change is not due to the decomposition of the pyrene moiety. We thus ascribe the fluorescent change to the photochemical reaction mainly of the 1, 2, 3-triazole units, which causes the release of the pyrene from PyP5 to thus recover the monomer fluorescence (Scheme S2 in Supporting information) [67].

|
Download:
|
| Fig. 3. (a) Fluorescence spectral change of 2.0 μmol/L solution of PyP5 in DCM upon irradiation at 365 nm with a UV lamp (λex = 330 nm). (b) UV–vis spectral change upon irradiation of 2.0 μmol/L PyP5 in DCM. (c) Fluorescence intensity change monitored at 379 nm and 473 nm of 2.0 μmol/L PyP5 in DCM upon irradiation at 365 nm with a UV lamp (λex = 330 nm). (d) The CIE chromaticity coordinate changes according to the fluorescence spectra. The inset showed the photographs of PyP5 in DCM before (A) and after (B) irradiation at 365 nm for 20 min. | |
Such an emission changes due to the photolysis of PyP5 inspired us to further explore its application in photo-writing. Thus, polyvinyl pyrrolidone (PVP) powders were added to a dichloromethane solution of PyP5 to give a transparent PVP/ PyP5 gel (Fig. 4a) [68]. Light green fluorescence in the gel was observed under excitation with a 365 nm lamp (Fig. 4b), suggesting PyP5 kept in its excimer form in the gel. Mask-illumination was applied by photoirradiation of the gel covered with corresponding pictures with the 365-nm LED (Fig. 4c). After the mask-illumination for 10 min, all the gels showed the excimer fluorescence of the masked picture, which were not observable under sunlight (Figs. 4d and e). As a comparison, the mask-illumination was carried out with a gel containing pyrene or R1, which, however, cannot show the similar photo-writing features. Thus, the gel containing PyP5 could be a confidential lithographic material with potential application for the secret photo-writing.

|
Download:
|
| Fig. 4. Procedures of passive photo-writing in a PVP/PyP5 gel contained in a petri dish for 10 min with a 365 nm UV lamp (150 mW/cm2). (a, b) Photograph of the PVP/ PyP5 gel under ambient light and under 365 nm UV lamp respectively; (c) Masked photo-writing procedure on the PVP/PyP5 gel. (d, e) The photograph of PVP/PyP5 gel under 365 nm UV lamp and ambient light, respectively, after the masked photowriting. | |
In conclusion, we synthesized a rim-differentiated C5-symmetric tiara-pillar[5]arene PyP5 in which 5 pyrenes were oriented at the same rim of the pillar[5]arene. PyP5 showed excimer fluorescence overwhelmingly higher than the monomer fluorescence even at the highly diluted solution as a result of the confined template effect of the pillararene framwork. The excimer fluorescence could be switched to monomer fluorescence by photoirradiation due to the photodecomposition of PyP5, which opened up new possibility for the application of secret photo-writing. The present study provided a new aspect of the application of pillar[n]arenes by virtue of the highly ordered arrangement of the functional groups anchored on the rims, and should open a new avenue for the application of pillar [n]arenes.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsWe acknowledge the support of this work by National Key Research and Development Program of China (No. 2017YFA0505903), the National Natural Science Foundation of China (Nos. 21871194, 21971169, 21572142), Science & Technology Department of Sichuan Province (Nos. 2019YJ0160, 2019YJ0090), Comprehensive Training Plat form of Specialized Laboratory, College of Chemistry and Prof.Peng Wu of Analytical & Testing Center, Sichuan University.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.079.
| [1] |
T. Ogoshi, T.A. Yamagishi, Y. Nakamoto, Chem. Rev. 116 (2016) 7937-8002. DOI:10.1021/acs.chemrev.5b00765 |
| [2] |
J.R. Wu, A.U. Mu, B. Li, et al., Angew. Chem. Int. Ed. 57 (2018) 9853-9858. DOI:10.1002/anie.201805980 |
| [3] |
W.B. Hu, H.M. Yang, W.J. Hu, et al., Chem. Commun. 50 (2014) 10460-10463. DOI:10.1039/C4CC01810A |
| [4] |
B. Jiang, W. Wang, Y. Zhang, et al., Angew. Chem. Int. Ed. 56 (2017) 14438-14442. DOI:10.1002/anie.201707209 |
| [5] |
C. Li, K. Han, J. Li, et al., Org. Lett. 14 (2012) 42-45. DOI:10.1021/ol2027834 |
| [6] |
J.C. Gui, Z.Q. Yan, Y. Peng, et al., Chin. Chem. Lett. 27 (2016) 1017-1021. DOI:10.1016/j.cclet.2016.04.021 |
| [7] |
X.B. Hu, Z. Chen, L. Chen, et al., Chem. Commun. 48 (2012) 10999-11001. DOI:10.1039/c2cc36027f |
| [8] |
Y. Wang, J.F. Xu, Y.Z. Chen, et al., Chem. Commun. 50 (2014) 7001-7003. DOI:10.1039/C4CC02760D |
| [9] |
N.L. Strutt, R.S. Forgan, J.M. Spruell, et al., J. Am. Chem. Soc. 133 (2011) 5668-5671. DOI:10.1021/ja111418j |
| [10] |
N. Cheng, Y. Chen, X. Wu, et al., Chem. Commun. 54 (2018) 6284-6287. DOI:10.1039/C8CC03306D |
| [11] |
L. Chen, W. Si, L. Zhang, et al., J. Am. Chem. Soc. 135 (2013) 2152-2155. DOI:10.1021/ja312704e |
| [12] |
L. Ma, S. Wang, C. Li, et al., Chem. Commun. 54 (2018) 2405-2408. DOI:10.1039/C8CC00213D |
| [13] |
J. Chen, H. Ni, Z. Meng, et al., Nat. Commun. 10 (2019) 3546. DOI:10.1038/s41467-019-11553-7 |
| [14] |
W.B. Hu, Z. Wang, X.L. Zhao, et al., Chem. Eur. J. 25 (2019) 2189-2194. |
| [15] |
J. Ji, Y. Li, C. Xiao, et al., Chem. Commun. 56 (2020) 161-164. DOI:10.1039/C9CC08541F |
| [16] |
C. Fan, J. Yao, G. Li, et al., Chem. Eur. J. 25 (2019) 12526-12537. DOI:10.1002/chem.201902676 |
| [17] |
K. Yang, S. Chao, F. Zhang, et al., Chem. Commun. 55 (2019) 13198-13210. DOI:10.1039/C9CC07373F |
| [18] |
J. Yao, W. Wu, W. Liang, et al., Angew. Chem. Int. Ed. 56 (2017) 6869-6873. DOI:10.1002/anie.201702542 |
| [19] |
L. Chen, Y. Cai, W. Feng, et al., Chem. Commun. 55 (2019) 7883-7898. DOI:10.1039/C9CC03292D |
| [20] |
Q. Zhao, Y. Chen, B. Sun, et al., Eur. J. Org. Chem. (2019) 3396-3400. |
| [21] |
R. Zhang, X. Yan, H. Guo, et al., Chem. Commun. 56 (2020) 948-951. DOI:10.1039/C9CC09155F |
| [22] |
B. Hua, L. Shao, Z. Zhang, et al., J. Am. Chem. Soc. 141 (2019) 15008-15012. DOI:10.1021/jacs.9b08257 |
| [23] |
Y. Zhu, L. Xu, L. Wang, et al., Chem. Commun. 55 (2019) 5910-5913. DOI:10.1039/C9CC02585E |
| [24] |
H. Zhang, X. Ma, J. Guo, et al., RSC Adv. 3 (2013) 368-371. DOI:10.1039/C2RA22123C |
| [25] |
S. Sun, X.Y. Hu, D. Chen, et al., Polym. Chem. 4 (2013) 2224-2229. DOI:10.1039/c3py00162h |
| [26] |
B. Hua, L. Shao, G. Yu, et al., Chem. Commun. 52 (2016) 10016-10019. DOI:10.1039/C6CC04919B |
| [27] |
H. Wang, C.N. Zhu, H. Zeng, et al., Adv. Mater. 31 (2019) 1807328. DOI:10.1002/adma.201807328 |
| [28] |
C. Fan, W. Wu, J.J. Chruma, et al., J. Am. Chem. Soc. 138 (2016) 15405-15412. DOI:10.1021/jacs.6b07946 |
| [29] |
L.B. Meng, D. Li, S. Xiong, et al., Chem. Commun. 51 (2015) 4643-4646. DOI:10.1039/C5CC00398A |
| [30] |
M. Hao, G. Sun, M. Zuo, et al., Angew. Chem. Int. Ed. 58 (2019) 2-8. DOI:10.1002/anie.201813331 |
| [31] |
K. Jie, M. Liu, Y. Zhou, et al., J. Am. Chem. Soc. 139 (2017) 2908-2911. DOI:10.1021/jacs.6b13300 |
| [32] |
T. Ogoshi, R. Sueto, Y. Hamada, et al., Chem. Commun. 53 (2017) 8577-8580. DOI:10.1039/C7CC04454B |
| [33] |
K. Jie, Y. Zhou, E. Li, et al., J. Am. Chem. Soc. 140 (2018) 3190-3193. DOI:10.1021/jacs.7b13156 |
| [34] |
Y. Li, Y. Shu, X. Wang, et al., Chem. Commun. 55 (2019) 6301-6304. DOI:10.1039/C9CC02352F |
| [35] |
S. Sarkar, S. Roy, A. Sikdar, et al., Analyst 138 (2013) 7119-7126. DOI:10.1039/c3an00928a |
| [36] |
S. Long, Q. Qiao, F. Deng, et al., Chem. Commun. 55 (2019) 969-972. DOI:10.1039/C8CC09544B |
| [37] |
J.B. Birks, L.G. Christophorou, Spectrochim. Acta 19 (1963) 401-410. DOI:10.1016/0371-1951(63)80051-X |
| [38] |
P. Wanat, R. Kasprzyk, M. Kopcial, et al., Chem. Commun. 54 (2018) 9773-9776. DOI:10.1039/C8CC04968H |
| [39] |
T.M. Figueira-Duarte, K. Müllen, Chem. Rev. 111 (2011) 7260-7314. DOI:10.1021/cr100428a |
| [40] |
J. Wu, W. Liang, T. Niu, et al., Chem. Commun. 54 (2018) 9206-9209. DOI:10.1039/C8CC03660H |
| [41] |
M. Nakamura, M. Fukuda, T. Takada, et al., Org. Biomol. Chem. 10 (2012) 9620-9626. DOI:10.1039/c2ob26773j |
| [42] |
H.J. Kim, J. Hong, A. Hong, et al., Org. Lett. 10 (2008) 1963-1966. DOI:10.1021/ol800475d |
| [43] |
P. Conlon, C.J. Yang, Y. Wu, et al., J. Am. Chem. Soc. 130 (2008) 336-342. DOI:10.1021/ja076411y |
| [44] |
K. Kawai, H. Yoshida, T. Takada, et al., J. Phys. Chem. B 108 (2004) 13547-13550. DOI:10.1021/jp049543b |
| [45] |
B. Schazmann, N. Alhashimy, D. Diamond, J. Am. Chem. Soc. 128 (2006) 8607-8614. DOI:10.1021/ja061917m |
| [46] |
Z. Zhao, S. Chen, J.W.Y. Lam, et al., J. Mater. Chem. 21 (2011) 7210-7216. DOI:10.1039/c0jm04449k |
| [47] |
J.S. Yang, C.S. Lin, C.Y. Hwang, Org. Lett. 3 (2001) 889-892. DOI:10.1021/ol015524y |
| [48] |
S. Karuppannan, J.C. Chambron, Chem. Asian J. 6 (2011) 964-984. DOI:10.1002/asia.201000724 |
| [49] |
X.L. Ni, S. Wang, X. Zeng, et al., Org. Lett. 13 (2011) 552-555. DOI:10.1021/ol102914t |
| [50] |
J. Cho, T. Pradhan, J.S. Kim, et al., Org. Lett. 15 (2013) 4058-4061. DOI:10.1021/ol401469z |
| [51] |
J. Huang, Y. Wu, Y. Chen, et al., Angew. Chem. Int. Ed. 50 (2011) 401-404. DOI:10.1002/anie.201005375 |
| [52] |
J. Ji, W. Wu, W. Liang, et al., J. Am. Chem. Soc. 141 (2019) 9225-9238. DOI:10.1021/jacs.9b01993 |
| [53] |
X. Wei, W. Wu, R. Matsushita, et al., J. Am. Chem. Soc. 140 (2018) 3959-3974. DOI:10.1021/jacs.7b12085 |
| [54] |
J. Yao, Z. Yan, J. Ji, et al., J. Am. Chem. Soc. 136 (2014) 6916-6919. DOI:10.1021/ja5032908 |
| [55] |
Z. Yan, Q. Huang, W. Liang, et al., Org. Lett. 19 (2017) 898-901. DOI:10.1021/acs.orglett.7b00057 |
| [56] |
M. Rao, K. Kanagaraj, C. Fan, et al., Org. Lett. 20 (2018) 1680-1683. DOI:10.1021/acs.orglett.8b00520 |
| [57] |
H. Lai, T. Zhao, Y. Deng, et al., Chin. Chem. Lett. 30 (2019) 1979-1983. DOI:10.1016/j.cclet.2019.09.009 |
| [58] |
J. Yi, W. Liang, X. Wei, et al., Chin. Chem. Lett. 29 (2018) 87-90. DOI:10.1016/j.cclet.2017.05.004 |
| [59] |
J. Ding, J. Chen, W. Mao, et al., Org. Biomol. Chem. 15 (2017) 7894-7897. DOI:10.1039/C7OB02013A |
| [60] |
M. Guo, X. Wang, C. Zhan, et al., J. Am. Chem. Soc. 140 (2018) 74-77. DOI:10.1021/jacs.7b10767 |
| [61] |
D. Niu, Y. Jiang, L. Ji, et al., Angew. Chem. Int. Ed. 58 (2019) 5946-5950. DOI:10.1002/anie.201900607 |
| [62] |
J.K. Choi, S.H. Kim, J. Yoon, et al., J. Org. Chem. 71 (2006) 8011-8015. DOI:10.1021/jo060981j |
| [63] |
P.L. Piciulo, J.K. Thomas, J. Chem. Phys. 68 (1978) 3260-3264. DOI:10.1063/1.436130 |
| [64] |
C.D. Borsarelli, H.A. Montejano, J.J. Cosa, et al., J. Photochem. Photobiol. A: Chem. 91 (1995) 13-19. DOI:10.1016/1010-6030(95)04124-X |
| [65] |
T.L. Gilchrist, G.E. Gymer, Adv. Heterocycl. Chem. 16 (1974) 33-85. |
| [66] |
G. Maier, J. Eckwert, A. Bothur, et al., Liebigs. Ann. (1996) 1041-1053. |
| [67] |
N.A. Al-Jalal, N.A. Al-Awadi, M.R. Ibrahim, et al., Intl. J. Chem. 5 (2013) 80. |
| [68] |
J. Lin, S. Wan, W. Liu, et al., Chem. Commun. 55 (2019) 4299-4302. DOI:10.1039/C9CC01388A |
 2021, Vol. 32
2021, Vol. 32 


