b College of Pharmacy, Yantai University, Yantai 264005, China
Copper, a required redox-active nutrient for life, is an essential element that plays important roles in many critical physiological and pathological processes, including oxidative stress protection, hormone production, mitochondrial respiration, bone metabolism, wound healing, connective tissue formation and iron transportation [1, 2]. The dysregulation of cellular copper is related to many severe diseases such as cancer [3, 4], cardiovascular disorders [5], Alzheimer's disease (AD) [6], Menkes (MS) [7], obesity and diabetes [8, 9]. Reliable detection and monitoring of copper is extremely important in living biosystems for the global physiological and/or pathological consequences of copper regulation [10-12]. Fluorescence imaging with designed probes has been recognized as one of the most non-invasive and versatile approaches for tracking the sophisticated structure and activities in living cells. Fluorescent probes with substantial photostability, sensitivity and selectivity are the key for fluorescence imaging of complicated bioactivities in vivo. So far, numerous fluorescent probes for monitoring Cu+ in living cells have been developed, and some of them are near-infrared (NIR) probes with advantaged tissue penetration [13, 14]. Most of these probes are organic fluorophores with sufficient lipophilicity that ensures the efficient diffusion of probes communicating across plasma membrane. However, high lipophilicity usually brings poor solubility and results in the formation of colloidal aggregation in aqueous solution. Formation of aggregates usually causes quenching (ACQ) effect and dramatically alters their photophysical properties, impeding their applications in biological applications in vivo [15].
Copper-responsive triarylpyrazoline (CTAP) is a group of well explored Cu+-selective fluorescent probes with tetrathiaza crown-as the copper-selective receptor based on Photoinduced electron transfer (PeT) mechanism. However, the first CTAP of this family, CTAP-1 presents as colloidal aggregates of several nanometers (50–60 nm) in aqueous solution, potentially affecting their applications [12]. CTAP-2, a hydrophilic triarylpyraziline-based probe with outstanding solubility has proved to be a sensitive probe for monovalent copper bound to metallochaperone Atox1 in gel electrophoresis [16]. Nonetheless, they still have some limitations in imaging of Cu+ in vivo, such as short absorption and emission wavelengths. Recently, substitution of bridging oxygen atom by silicon in rhodamine (SiR) has attracted extensive attention as a novel family of NIR dyes with high brightness and photostability [17-20]. Furthermore, the fluorescence of SiR can be efficiently regulated via a PeT strategy [21]. And SiR has been proved to be suitable for design of PeT-based probes, such as CaSiR [22]. Herein, we have introduced the hydrophilic triarylpyraziline group into Si-rhodamine for monitoring intracellular Cu+ in vivo. Although SiR is typically believed to form aggregates in aqueous solutions, the resulted probe, SiR-Cu (Scheme 1) is highly hydrophilic.
|
Download:
|
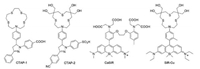
| Scheme 1. Chemical structures of CTAP-1, CTAP-2, CaSiR and SiR-Cu. | |
The synthesis of probe SiR-Cu is outlined in Scheme 2 and Scheme S1 (Supporting information). Firstly, a tetra hydroxymethyl modified thiazacrown (TAC-1) was prepared and further combined with Si-containing xanthone (SiX) [23], followed by deprotection in HCl solution to yield the probe SiR-Cu. The structure of probes SiR-Cu and important intermediates were confirmed by high-resolution mass spectrometry and NMR spectroscopy.

|
Download:
|
| Scheme 2. Synthesis of probe SiR-Cu. | |
The resulted probe SiR-Cu can be readily dissolved in aqueous MOPS solution with bright blue color (Fig. S1 in Supporting information). Next, we evaluated its spectral properties in the absence or presence of Cu+ in the MOPS/K+ buffer (pH 7.2, [MOPS] = 25 mmol/L). In the aqueous solution, SiR-Cu exhibited a strong characteristic absorption at 656 nm (ε = 68, 000 L mol−1 cm−1, Fig. S2 in Supporting information) with weak emission (Φ = 0.01). Upon addition of Cu+, the intensity of fluorescence with maxima at 680 nm gradually increased without affecting the intensity of absorption spectra, presumably due to the inhibition of the PeT process by the complexation of thiazacrown group and Cu+ (Fig. 1a and Fig. S2 in Supporting information). To confirm the strong chelation of thiazacrown with Cu+, 1H NMR titration was carried out and showed the progressive shift in the protons of the thiazacrown and the aromatic proton adjacent to the crown moiety with the addition of Cu+ (Fig. S3 in Supporting information). Fluorescence titration of probe SiR-Cu with Cu+ showed a linear increasing in emission with a sharp saturation when the concentration of Cu+ reached 1.0 equiv. (LOD =1.2 nmol/L, Fig. 1b), indicating a 1:1 binding model between the probe and Cu+. The intensity of fluorescence reached 23-fold (Φ = 0.18) with extinction coefficient of 60, 000 L mol−1 cm−1 at saturation of Cu+. The 1:1 host-guest stoichiometry is further confirmed by the Job's plot analysis (Fig. S4 in Supporting information). To determine the apparent dissociation constant (Kd) for the complex, a competitive fluorescence titration by using thiourea as ligand was applied (Figs. S5 and S6 in Supporting information). The Kd value is calculated to be 14 ± 2 pmol/L, suggesting the developed probe SiR-Cu has high affinity and sensitivity for detecting trace amounts of Cu+.

|
Download:
|
| Fig. 1. (a) Fluorescence response of the probe SiR-Cu (2.5 μmol/L) in the presence of different equiv. of Cu+ (0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.2, 1.4, 1.6, 1.8, 2.0) in MOPS/K+ buffer (pH 7.2, [MOPS] =25 mmol/L). (b) Fluorescence intensity ratio (F/F0) changes at 680 nm. Excitation at 620 nm. | |
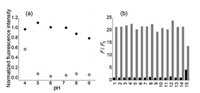
The effects of pH on the fluorescence of probe SiR-Cu and its response to Cu+ were evaluated in detail. Within pH 5.0–8.0, probe SiR-Cu exhibits reliable response to Cu+ (Fig. 2a), satisfying the fluorescence imaging applications in vivo. In acidic conditions (pH < 4.0), fluorescence released from the probe, resulting in strong background signal. Whereas in basic solution (pH > 9.0), the fluorescence intensity from the fluorescent probe-Cu+ complex got decreased. Furthermore, the fluorescence response of probe SiR-Cu was proved to be highly selective toward Cu+ (Fig. 2b) with ignorable effects by other biologically relevant ions except for Hg2+, which could occupy the chelating site of the thiosemicarbazide moiety and hinder the further chelation with Cu+. Since the concentration of intracellular Hg2+ ion is negligible, this probe displays high specificity and durable fluorescence for detect the intracellular Cu+. Moreover, water-soluble probes usually suffered from poor cellular permeability, herein, substantial cellular permeability of SiR-Cu was confirmed by fluorescence imaging of HepG2 cells incubated with both probe and analyses (Fig. 3). Additionally, MTT assays revealed that the cellular viabilities of HepG2 cells were not noticeably affected by incubation with 1∼20 μmol/L probe SiR-Cu for 24 h (Fig. S7 in Supporting information). Thus, the probe SiR-Cu meets the requirements for imaging Cu+ in vivo, including high sensitivity toward Cu+, reliable response at physiological pH, explicit specificity and low cytotoxicity. These desirable characters prompted us to evaluate the ability of the probe towards fluorescence imaging of subcellular Cu+ in living cells.
|
Download:
|
| Fig. 2. (a) Effect of pH on the probe SiR-Cu (○) and its fluorescent responses to Cu+ (•). (b) Fluorescence responses of probe SiR-Cu to various metal ions in MOPS/K+ buffer (pH 7.2, [MOPS] = 25 mmol/L). Black bars: probe SiR-Cu in the presence of an excess of the metal ions (25 μmol/L for Cu2+, 2.5 μmol/L for Hg2+ and 250 μmol/L for other cations); gray bars: addition of 2.5 μmol/L Cu+ to the solution of probe SiR-Cu containing other potential interference metal ions (1. only probe, 2. Na+, 3. Ca2+, 4. Mg2+, 5. Zn2+, 6. Cd2+ 7. Mn2+, 8. Pd2+, 9. Fe2+, 10. Cr3+, 11. Ba2+, 12. Cu2+, 13. Co2+, 14. Ni2+, 15. Hg2+). | |
|
Download:
|
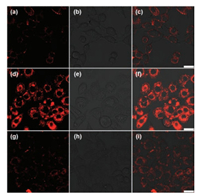
| Fig. 3. Fluorescence images of living HepG2 cells. (a-c) Fluorescence image of the cells treated with the probe (5 μmol/L) for 10 min; (d-f) Fluorescence image of the cells pre-treated with CuCl2 (200 μmol/L) for 7 h and further incubated with the probe (5 μmol/L) for 10 min; (g-i) Fluorescence image of the cells pre-treated with CuCl2 (200 μmol/L) for 7 h, further incubated with probe (5 μmol/L) for 10 min, and subsequently treated with BETA (500 μmol/L) for another 10 min. | |
The HepG2 cells stained with probe SiR-Cu (5 μmol/L) showed weak fluorescence emission (Figs. 3a-c). For the second group of cells, we pre-treated the cells with Cu2+ which can be converted into reduced Cu+ under the reducing environment of the cytosol. The pre-treated cells were further incubated with probe SiR-Cu, showing bright red fluorescence (Figs. 3d-f). To further confirm that the above observed red fluorescence were indeed induced by Cu+ ions, the third group of cells were pre-treated with CuCl2 (200 μmol/L) for 7 h, further incubated with probe (5 μmol/L) for 10 min, and subsequently treated with a competing Cu+ chelator BETA (500 μmol/L) for another 10 min. As shown in Figs. 3g-i, almost no fluorescence was observed in the BETA-treated cells. The results indicate that the water-soluble probe SiR-Cu can readily permeate the lipophilic cell membrane and selectively detect intracellular Cu+ with reliable sensitivity.
Based on the above promising results of imaging exogenous Cu+ in the living cells, we further investigated the feasibility of the probe SiR-Cu to imaging endogenous Cu+ in living cells. Ascorbic acid can facilitate the releasing of labile endogenous Cu+ ions in living cells. The HepG2 cells stained with ascorbic acid (1 mmol/L) for 4 h and then incubated with 5 μmol/L probe SiR-Cu for 10 min at 37 ℃ are highly fluorescent (Figs. 4a-c). Moreover, the bright red fluorescence can be quenched by further incubation of the cells with 500 μmol/L competing Cu+ chelator BETA (Figs. 4d-f). Thus, above experimental results have established that SiR-Cu is a sensitive fluorescent probe for monitoring both endogenous and exogenous subcellular Cu+ in living cells, suggesting the potential utility of the developed probe SiR-Cu in investigating the roles of Cu+ in the critical physiological and pathological processes.

|
Download:
|
| Fig. 4. Fluorescence images of living HepG2 cells. (a-c) Fluorescence image of the cells pre-treated with ascorbic acid (1 mmol/L) for 4 h and then incubated with the probe (5 μmol/L) for 10 min; (d-f) Fluorescence image of the cells pre-treated with ascorbic acid (1 mmol/L) for 4 h, further incubated with the probe (5 μmol/L) for 10 min, and subsequently treated with the competing Cu+ chelator BETA (500 μmol/L) for an additional 10 min. | |
In summary, a hydrophilic triarylpyraziline-based NIR fluorescent probe SiR-Cu was developed for tracking trace amount of Cu+ with reliable selectivity and sensitivity. Owing to the critical roles that Cu+ plays in biosystems, monitoring the activity of Cu+ in vivo have attracted extensive attention, although there are still many limitations in the current library of probes/sensors for Cu+. The turn-on fluorescence probe, SiR-Cu can be directly dissolved into water with substantial cellular permeability. The mechanism for recognition of Cu+ was evaluated using 1H NMR and fluorescence spectroscopy. The developed probe exhibits reliable fluorescence response to Cu+ at physiological pH. In addition, confocal microscopy analysis with probe SiR-Cu demonstrated that this probe can sensitively monitor both the exogenous and endogenous Cu+ ions in living cells. The probe affords a good level of cellular uptake, reliable emission stability and low cytotoxicity. Taking advantage of the promising characteristics of the probe SiR-Cu, we expect that the novel probe may find extensive applications in the physiological and pathological consequences of copper regulation in living systems.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21602250, 81830106) and Natural Science Foundation of Shanghai (Nos. 19ZR1480000, 20ZR1470200).
Appendix A. Supplementary dataSupplementary material relatedto this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.11.032.
| [1] |
B.E. Kim, T. Nevitt, D.J. Thiele, Nat. Chem. Biol. 4 (2008) 176-185. DOI:10.1038/nchembio.72 |
| [2] |
E.D. Harris, Crit. Rev. Cl. Lab. Sci. 40 (2003) 547-586. DOI:10.1080/10408360390250649 |
| [3] |
T. Finkel, M. Serrano, M.A. Blasco, Nature 448 (2007) 767-774. DOI:10.1038/nature05985 |
| [4] |
D.C. Brady, M.S. Crowe, M.L. Turski, et al., Nature 509 (2014) 492-496. DOI:10.1038/nature13180 |
| [5] |
R. Nath, Int. J. Biochem. Cell Biol. 29 (1997) 1245-1254. DOI:10.1016/S1357-2725(97)00060-5 |
| [6] |
A.I. Bush, Trends Neurosci. 26 (2003) 207-214. DOI:10.1016/S0166-2236(03)00067-5 |
| [7] |
Z. Tumer, L.B. Moller, Eur. J. Hum. Genet. 18 (2010) 511-518. DOI:10.1038/ejhg.2009.187 |
| [8] |
D. Huster, S. Lutsenko, Mol. Biosyst. 3 (2007) 816-824. DOI:10.1039/b711118p |
| [9] |
T.S. Nielsen, N. Jessen, J.O. Jorgensen, N. Moller, S. Lund, J. Mol. Endocrinol. 52 (2014) 199-222. DOI:10.1530/JME-13-0277 |
| [10] |
D.W. Domaille, E.L. Que, C.J. Chang, Nat. Chem. Biol. 4 (2008) 168-175. DOI:10.1038/nchembio.69 |
| [11] |
C.Y. Chung, J.M. Posimo, S. Lee, et al., Proc. Natl. Acad. Sci. U. S. A. 116 (2019) 18285-18294. DOI:10.1073/pnas.1904610116 |
| [12] |
L. Yang, R. McRae, M.M. Henary, et al., Proc. Natl. Acad. Sci. U. S. A. 102 (2005) 11179-11184. DOI:10.1073/pnas.0406547102 |
| [13] |
A.F. Chaudhry, S. Mandal, K.I. Hardcastle, C.J. Fahrni, Chem. Sci. 2 (2011) 1016-1024. DOI:10.1039/c1sc00024a |
| [14] |
J.J.A. Cotruvo, A.T. Aron, K.M. Ramos-Torres, C.J. Chang, Chem. Soc. Rev. 44 (2015) 4400-4414. DOI:10.1039/C4CS00346B |
| [15] |
Y. Huang, J. Xing, Q. Gong, et al., Nat. Commun. 10 (2019) 169. |
| [16] |
M.T. Morgan, P. Bagchi, C.J. Fahrni, J. Am. Chem. Soc. 133 (2011) 15906-15909. DOI:10.1021/ja207004v |
| [17] |
M. Fu, Y. Xiao, X. Qian, D. Zhao, Y. Xu, Chem. Commun. 1780 (2008) 1782. |
| [18] |
Y. Koide, Y. Urano, K. Hanaoka, et al., J. Am. Chem. Soc. 134 (2012) 5029-5031. DOI:10.1021/ja210375e |
| [19] |
M. Li, Y. Li, X. Wang, X. Cui, T. Wang, Chin. Chem. Lett. 30 (2019) 1682-1688. DOI:10.1016/j.cclet.2019.06.036 |
| [20] |
Y. Xiao, X. Qian, et al., Coord. Chem. Rev. 423 (2020) 213513. |
| [21] |
Y. Koide, Y. Urano, K. Hanaoka, T. Terai, T. Nagano, ACS Chem. Biol. 6 (2011) 600-608. DOI:10.1021/cb1002416 |
| [22] |
T. Egawa, K. Hanaoka, Y. Koide, et al., J. Am. Chem. Soc. 133 (2011) 14157-14159. DOI:10.1021/ja205809h |
| [23] |
T. Wang, Q.J. Zhao, H.G. Hu, et al., Chem. Commun. 48 (2012) 8781-8783. DOI:10.1039/c2cc34159j |
 2021, Vol. 32
2021, Vol. 32 

