Currently, six-membered phosphacycle based fluorophores have attracted considerable attention in a variety of fields, ranging from coordination chemistry [1] to optical material science [2]. Among these six-membered phosphacycles, λ5-phosphinine is one of the less-studied areas since most of the known λ5-phosphinines are nonemissive [3]. Although the recent results highlighted the fascinating optical and electronic properties of λ5-phosphinines, but the efficient methods to access luminescent λ5-phosphinines are very limited [4, 2c, 2d]. Matveeva and Kostyuk developed a series of heterocyclization of phosphonium ylides derivatives with acetylenes to give λ5-phosphinines [5, 3]. But the photophysical chemistry of these λ5-phosphinines has never been discussed. Several fluorescent 2, 4, 6-triaryl-λ5-phosphinines were synthesized [6, 2b, 2d] and Müller developed the first blue λ5-phosphinine emitter based OLED very recently [7]. After diligent studies on a serendipitous result, Hayashi discovered simple access to a new tunable fluorophore, 2, 6-dicyano-λ5-phosphinine [2c]. It will be useful to develop new methods for the construction of diversified luminescent λ5-phosphinines with the hypothesis that structural diversity will lead to diverse photophysical properties.
Herein, we present a facile, high-yielding protocol to luminescent λ5-phosphanaphthalenes that relies on the intramolecular Michael addition of the in-situ formed zwitterionic carbenoid species to aldehyde.
Recently, our group developed a series of intramolecular ring-closing reactions for the synthesis of annulated six-membered phosphacycles [8]. Kwon and co-workers reported an interesting three-component coupling reaction of trialkylphosphines, methyl phenylpropiolate, and 4-pyridinecarboxaldehyde to give stable tetravalent phosphonium enolate zwitterions [9]. Thus, we envisioned that ortho-phosphinobenzoaldehydes would react with an electron-deficient alkyne DMAD to form zwitterionic phosphonium heterocycle 2, which would eventually lead to the formation of λ5-phosphanaphthalenes 3 via an intramolecular addition to a carbonyl group (Scheme 1) [10]. A clean reaction of 2-diphenylphosphinobenzaldehyde with DMAD was observed by 31P NMR (5 ppm) spectroscopy. However, neither starting material nor product was obtained after workup and purification.
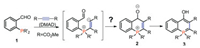
|
Download:
|
| Scheme 1. Proposed reaction of 1 with electron-deficient alkyne. | |
We hypothesized that the reaction provided the desired phosphacycle 3, the keto/enol tautomerization gave the unstable 3′ in Scheme 2 which decomposed during the workup procedure. If the hydrogen transfer process is blocked by alkylation of the hydroxy group of 3, the stable λ5-phosphanaphthalene 4 might be isolated (Scheme 2).

|
Download:
|
| Scheme 2. Tautomerization of phosphanaphthalenes. | |
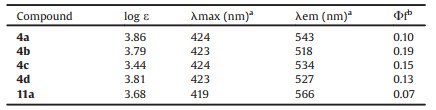
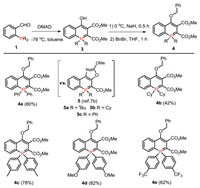
An alkylation procedure was added after the formation of zwitterionic phosphonium 3 from the reaction of ortho-phosphinobenzoaldehyde with DMAD. The THF solution of the crude reaction mixture was treated with NaH (3 equiv.) at 0 ℃ for half-hour, then benzyl bromide (4 equiv.) was added, and the reaction mixture was refluxed for 1 h. It is gratifying to see the desired λ5-phosphinine 4a was isolated in 80% yield after chromatography (Fig. 1). A series of 4-alkyloxyl-λ5-phosphinines was obtained (Scheme 3). Higher reaction efficiencies were observed with neutral (4a, 4c) or electron-rich (4d) aryl-substituted phosphine than electron-deficient aryl-substituted 4e. A low yield was obtained with cyclohexyl substituted 4b. All of these λ5-phosphanaphthalenes are luminescent under UV light excitation.

|
Download:
|
| Fig. 1. X-ray crystal structure of 4a. The level set for thermal ellipsoids of all atoms is 30%. All the hydrogen atom have been omitted for clarity. Main bond lengths (Å) and angles (deg): C13-C18 1.411(3), C19-C20 1.358(3), C21-C20 1.446(3), C1-P1 1.809(2), P1-C7 1.821(2), P1-C13 1.791(2), P1-C21 1.734(2), C1-P1-C7 107.95(11), C13-P1-C1 107.66(11), C13-P1-C7 107.11(11), C21-P1-C1 114.57(11), C21-P1-C7 113.63(12), C21-P1-C13 105.49(12). CCDC: 1999936. | |
|
Download:
|
| Scheme 3. Synthesis of phosphanaphthalenes. | |
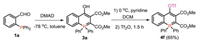
It should be noted that replacing NaH and benzyl bromide with pyridine and Tf2O provided trifluoromethanesulfonate (OTf) substituted 4f (Scheme 4), and the transition metal catalyzed cross-coupling with 4f is currently underway.
|
Download:
|
| Scheme 4. Synthesis of 4f. | |
The X-ray crystal structure analysis showed 4a containing a well delocalized cyclic ylidic structure with a small torsion angle (C20-C21-P1-C13, 6.2°(2)). The P-C(CO2Me) bond (P1-C21 1.734(2) Å) is shorter than that of the intracyclic P-C(Ph) bond (P1-C13 1.791(2) Å), while the bond distances of the phosphacyle are ranged from 1.358 Å to 1.446 Å. These data indicated that the luminescent 4a has a more planar phosphacycle and much shorter P-C(CO2Me) bond length than those of nonemissive 8a and 9. We have found that the photophysics of λ5-phosphanaphthalenes 5 (Scheme 3) [7b] depends significantly upon the nature of the exocyclic substituents on phosphorus, because the luminescent bulky alkyl, tBu (5a) and Cy (5b), -substituted products are more planar than the nonemissive phenyl substituted 5c. It should be noted that the phenyl substituted 4a is also more planar than the tricyclic fused analogue 5c, which indicates that structural and photophysical features of λ5- phosphanaphthalenes can be tuned by not only the exocyclic substituents on phosphorus but also the substitution pattern of the phospha-ring.
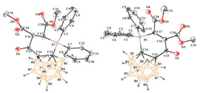
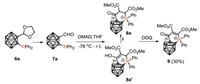
Recently, the three-dimensional (3D) aromaticity, electron-withdrawing property, and the formation of negatively charged nido-carborane via deboronation endow the ortho-carborane with many unique advantages as a building block [11, 12a, 12b]. Therefore, it would be interesting to replace the aryl with a carboranyl backbone. Surprisingly, the reaction of phosphinocarboranylaldehyde with DMAD provided different results. A stable white solid was isolated without alkylation. The 31P NMR analysis indicated that the white solid containing two types of phosphorus compounds, the major one exhibits a resonance at 28 ppm, while the minor one has a chemical shift of 14 ppm. Fortunately, the structure of the major product 8a was solved by the crystal analysis (Fig. 2). Since the mixture has one 1H NMR resonance at 13 ppm, a putative enol tautomer 8a' was assigned for the minor product in the mixture (Scheme 5).
|
Download:
|
| Fig. 2. X-ray crystal structure of 8a (left). The level set for thermal ellipsoids of all atoms is 30%. Except for the hydrogen atom on the carborane, it has been omitted for clarity. Main bond lengths (Å) and angles (deg): C13-C14 1.576(3), C14-C15 1.486(3), C15-C16 1.520(3), C16-C17 1.547(3), C1-P1 1.792(2), P1-C7 1.789(2), P1-C17 1.834(2), P1-C13 1.787(2), C1-P1-C17 105.91(10), C7-P1-C1 109.64(10), C1-P1-C13 112.87(10), C17-P1-C7 112.87(10), C7-P1-C13 110.59(10), C17-P1-C13 106.72(10), C14-C13-P1 118.51(15). CCDC: 1999934. X-ray crystal structure of 9 (right). The level set for thermal ellipsoids of all atoms is 30%. Except for the hydrogen atom on the carborane, it has been omitted for clarity. Main bond lengths (Å) and angles (deg): C13-C14 1.570(3), C14-C15 1.479(3), C15-C16 1.512(3), C16-C17 1.339(3), C1-P1 1.791(2), P1-C7 1.789(3), P1-C17 1.802(3), P1-C13 1.785(2), C1-P1-C17 108.42(12), C7-P1-C1 111.10(12), C7-P1-C17 108.46(12), C13-P1-C1 112.72(12), C13-P1-C7 109.24(11), C13-P1-C17 106.72(12), C14-C13-P1 117.43(17). CCDC: 1999935. | |
|
Download:
|
| Scheme 5. The nido-carborane fused phosphacycles. | |
After treated with DDQ, the two tautomers were converted into a single product 9, which was fully characterized by multinuclear NMR spectroscopy, high-resolution mass spectrometry (HR-MS), and X-ray diffraction. As shown in the X-ray crystal structure (Fig. 2), 9 has a much shorter distance (C16-C17 1.339(3) Å) between the two CO2Me groups than that (1.486(3) Å) of 8a and a more planar phosphacycle. To the best of our knowledge, these compounds represent the first examples of carborane fused six-membered phosphacycle [12].
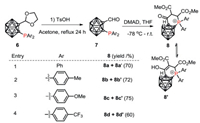
Replacing the phenyl substituents with electron-neutral methyl (Scheme 6, entry 2), electron-donating methoxy (entry 3), and electron-withdrawing CF3 (entry 4) substituted aryl groups provided similar tautomerized products, with keto tautomer as the major one. A little bit lower yield was obtained with CF3 (8d+8d', 60% isolated yield) substituted derivative.
|
Download:
|
| Scheme 6. Synthesis of zwitterionic nido-carborane fused P-heterocycles. | |
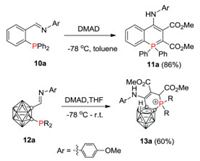
When the reaction was carried out with imino derivatives 10a, 4-amino-λ5-phosphinine 11a was obtained as a sole product directly without enamine to imine tautomerization. A similar phenomenon was observed with iminocarborane 12a (Scheme 7).
|
Download:
|
| Scheme 7. Synthesis of amino-substituted phosphacycles. | |
With these diverse λ5-phosphanaphthalenes molecules in hand, we next turned our attention to their photophysical properties by recording UV–vis and emission spectra in DCM at a low concentration (1 × 10-5 mol/L) as shown in Table 1 and Fig. 3. nido-Carborane fused phosphacycles 8a-d, 9 and 13a are nonemissive. Compared with 8a-d, λ5-phosphanaphthalenes 4a-d exhibit moderate fluorescent efficiency and bathochromic shift in their absorption wavelengths, due to the presence of an extended π-conjugation system. A low fluorescence quantum yield was observed with 4-amino-λ5-phosphinine 11a.
|
|
Table 1 Photophysical data of 4a, 4b, 4c, 4d, 11a. |

|
Download:
|
| Fig. 3. (a) UV–vis absorption of 4a, 4b, 4c, 4d, 11a in CH2Cl2 (1 × 10-5 mol/L) at 298 K. (b) PL spectra of 4a, 4b, 4c, 4d, 11a in CH2Cl2 (1 × 10-5 mol/L) at 298 K. | |
In summary, a one-pot reaction of easily accessible phosphine derivatives with DMAD was developed to synthesize the unknown zwitterionic nido-carborane fused six-membered phosphacycles and a new series of luminescent λ5-phosphanaphthalenes. This new result also highlighted the planarity of λ5-phosphinine is crucial to their luminescence, which can be tuned by the exocyclic substituents on phosphorus and substitution pattern of the ring. Further applications of this synthetic method and utilizations of the luminescent λ5-phosphanaphthalenes are underway in our group.
Declaration of competing interestThe authors declare that there are no conflicts of interest.
AcknowledgmentsWe are grateful to the National Natural Science Foundation of China (Nos. 21672193, 21272218), Ministry of Industry and Information Technology of the People's Republic of China (No. Z135060009002), 111 Project (No. D20003) and Zhengzhou University of China for financial support of this research.
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.11.018.
| [1] |
P.L. Floch, Coord. Chem. Rev. 250 (2006) 627-681. DOI:10.1016/j.ccr.2005.04.032 |
| [2] |
(a) A. Fukazawa, Y. Sato, T. Higashiyama, et al., Chem. Commun. (Camb.) 52(2016) 1120-1123; (b) C. Müller, D. Wasserberg, D. Vogt, et al., Chem. Eur. J. 13(2007) 4548-4559; (c) N. Hashimoto, R. Umano, O. Yusuke, et al., J. Am. Chem. Soc. 140(2018) 2046-2049; (d) A. Belyaev, Y. Chen, Z. Liu, et al., Chem. Eur. J. 25(2019) 6332-6341; (e) W. Ye, X. Li, H. Tian, et al., J. Org. Chem. 85(2020) 3879-3886; (f) P. Hindenberg, C. Romero-Nieto, Synlett 27(2016) 2293-2300. |
| [3] |
(a) E.D. Matveeva, D.S. Vinogradov, N.S. Zefirov, et al., Eur. J. Org. Chem. 2015(2015) 7324-7333; (b) E.D. Matveeva, T.A. Podrugina, N.S. Zefirov, et al., J. Org. Chem. 78(2013) 11691-11697; (c) E.D. Matveeva, T.A. Podrugina, N.S. Zefirov, et al., Tetrahedron 69(2013) 7395-7402; (d) E.D. Matveeva, T.A. Podrugina, N.S. Zefirov, et al., J. Org. Chem. 77(2012) 5770-5774; (e) E.D. Matveeva, T.A. Podrugina, N.S. Zefirov, et al., J. Org. Chem. 74(2009) 9428-9432; (f) A. Savateev, Y. Vlasenko, N. Shtil, A. Kostyuk, Eur. J. Inorg. Chem. 2016(2016) 628-632. |
| [4] |
(a) A. Belyaev, Y. Chen, S. Su, I.O. Koshevoy, et al., Chem. Commun. (Camb. ) 53(2017) 10954-10957; (b) P. Hindenberg, M. Busch, A. Paul, C. Romero-Nieto, et al., Angew. Chem. Int. Ed. 57(2018) 15157-15161; (c) T. Kato, T. Kuwabara, Y. Minami, T. Hiyama, Y. Ishii, Bull. Chem. Soc. Jpn. 92(2019) 1131-1141; (d) T. Delouche, A. Vacher, E. Caytan, P.A. Bouit, et al., Chem. Eur. J. 26(2020) 8226-8229. |
| [5] |
(a) Y.V. Svyaschenko, B.B. Barnych, D.M. Volochnyuk, N.V. Shevchuk, A.N. Kostyuk, J. Org. Chem. 76(2011) 6125-6133; (b) Y.V. Svyaschenko, A.N. Kostyuk, B.B. Barnych, D.M. Volochnyuk, Tetrahedron 63(2007) 5656-5664; (c) A.N. Kostyuk, Y.V. Svyaschenko, D.M. Volochnyuk, Tetrahedron 61(2005) 9263-6272. |
| [6] |
K. Dimroth, Delocalized posphorus-carbon double bonds, Heidelberg, Phosphorus-Carbon Double Bonds Fortschritte der Chemischen Forschung, 38/1, Springer, Berlin, 1973, doi: http://dx.doi.org/10.1007/BFb0051365.
|
| [7] |
G. Pfeifer, F. Chahdoura, M. Papke, et al., Chem. Eur. J. 26 (2020) 10534-10543. DOI:10.1002/chem.202000932 |
| [8] |
(a) L. Zhang, W. Yu, C. Liu, et al., Organometallics 34(2015) 5697-5702; (b) L. Zhang, F. Yang, G. Tao, et al., Eur. J. Inorg. Chem. 17(2017) 2355-2362; (c) H. Huang, Z. Wei, J. Hou, et al., Eur. J. Org. Chem. 22(2018) 2863-2869; (d) J. Hou, Y. Xu, Z. Gan, et al., J. Organomet. Chem. 879(2019) 158-161; (e) D. Wu, C. Hu, L. Qiu, Z. Duan, F. Mathey, Eur. J. Org. Chem. 37(2019) 6369-6376; (f) E. Si, P. Zhao, L. Wang, Z. Duan, F. Mathey, Eur. J. Org. Chem. 6(2020) 697-701. |
| [9] |
X.F. Zhu, C.E. Henry, O. Kwon, J. Am. Chem. Soc. 129 (2007) 6722-6723. DOI:10.1021/ja071990s |
| [10] |
A. Bhunia, T. Roy, R.G. Gonnade, A.T. Biju, Org. Lett. 16 (2014) 5132-5135. DOI:10.1021/ol502490t |
| [11] |
(a) A.R. Popescu, F. Teixidor, C. Viñas, Coord. Chem. Rev. 269(2014) 54-84; (b) M. Joost, L. Estévez, K. Miqueu, A. Amgoune, D. Bourissou, Angew. Chem. Int. Ed. 127(2015) 5325-5329; (c) Y.O. Wong, M.D. Smith, D.V. Peryshkov, Chem. Eur. J. 22(2016) 6764-6767; (d) J.A. Ioppolo, J.K. Clegg, L.M. Rendina, Dalton Trans. 20(2007) 1982-1985; (e) C.X. Cui, S. Ren, Z. Qiu, Z. Xie, Dalton Trans. 47(2018) 2453-2459; (f) L.E. Riley, A.J. Welch, Claire L. McMullin, et al., Dalton Trans. 46(2017) 5218-5228; (g) P. Coburger, E. Hey-Hawkins, et al., Inorg. Chem. 56(2017) 292-304. |
| [12] |
(a) A. Kreienbrink, M.B. Sárosi, E.G. Rys, P. Lönnecke, E. Hey-Hawkins, Angew. Chem. Int. Ed. 50(2011) 4701-4703; (b) A. Kreienbrink, M.B. Sárosi, R. Kuhnert, et al., Chem. Commun. (Camb. ) 51(2015) 836-838; (c) J. Schulz, A. Kreienbrink, P. Coburger, et al., Chem. Eur. J. 24(2018) 6208-6216; (d) G. Tao, Z. Duan, F. Mathey, Org. Lett. 21(2019) 2273-2276. |
 2021, Vol. 32
2021, Vol. 32