b Key Laboratory of Pesticide and Chemical Biology (CCNU), Ministry of Education, College of Chemistry, Central China Normal University, Wuhan 430079, China
Chirality has a marvelous impact on the various biological processes as most of the bio-related molecule display chirality [1]. A chiral molecule is non-superimposable on its mirror image and two non-identical mirror images are a pair of enantiomers. Enantiomers of chiral drugs show significant differences in terms of biochemical activity, influence, toxicity, and transport mechanism [2, 3]. Thus chiral recognition has always been a hot and exciting topic in chemical, biological, and pharmaceutical research. The improvement in methods to differentiate between two chiral isomers of a chiral drug is of importance in biotechnology, pharmaceutical science and technology [4-7]. In these efforts chiral HPLC (high-performance liquid chromatography) [8-10], GC (gas chromatography) [11, 12] and CE (capillary electrophoresis) [13, 14] were commonly used for chiral discrimination. However, these techniques have some drawbacks including time-consuming, inefficient, and required expansive instrumentation [15]. Therefore, a novel chiral discrimination platform capable of simple, low cost, and high sensitive recognition for chiral drug identification is highly preferred.
Up to now, many different gated materials with the capability of smart control ions and molecules transport have been broadly studied [16-19]. Compared with biological channels, solid-state synthetic bionic nanopores/nanochannels have attracted great attention due to their exceptional mechanical properties, controllable size and shape, good chemical stability, tunable porosity, and significant advantages for innovative applications, such as biosensing, filtering, energy utilization, and in controlling molecule transport [20-26]. For example, Li group have made great efforts to study the stimulus-response bionic nanochannel which can be regulated through temperature [27], light [28], pH [29] superior constituents [30, 31], etc. As compared to other methods, electrodes capable of chiral recognition bear obvious advantages of low cost, simple use and high sensitivity. Chiral electrochemical sensors have been reported based on the chirality of incorporating molecules including amino acids [29], proteins [32], and supramolecules onto the nanopore/nanochannel membranes surface provide an important tool for studying the molecule recognition and transportation [33-41]. Among various macrocyclic arenes, pillar[n]arenes, the most representative ones that possess a rigid macrocyclic frame, p-electron rich cavity, and man possibilities of easy functionalization, have appeared as a new star in supramolecular and host-guest chemistry since the discovery by Ogoshi and co-workers in 2008 [42]. The flexible functionalization of pillar[n]arenes constructively affords their possible usages in both organic and aqueous phases, making the host-guest interactions with a large variety of ionic or neutral guests possible [43]. A variety of pillar[n]arenes-based supramolecular assemblies have been reported, which have been widely used in molecular switches and molecular machines, metal–organic frameworks, controlled drug delivery systems, gas selective capture, sensors, and stimuli-responsive materials [44-50]. Due to these advantages of pillar[n]arenes, we proposed a new strategy for chiral response switchable nanochannel guarding electroactive ions transport through embedding the Pillar[5]arene based host-guest system into the nanochannel.
Herein, we have developed a simple and effective electrochemical chiral recognition method based on a chiral Pillar[5]arrene functionalized nanochannel, PC membrane, and gold electrode to study enantioselective recognition properties of propranolol drug (R/S-PPL). Chiral N-acetyl-l-cysteine decorated Pillar[5]arene (NALC-P5) was synthesized by the classical "thiol-ene" click reaction. The recognition performance was established by electrochemical methods based on its easy, quick and sensitive properties, and the mechanism of chiral R/S-PPL recognition was studied by NMR technique and contact angle measurements that directly correlated with the hydrophobicity of the nanochannel inner surface. After contact with the electrode, R/S-PPL molecules can diffuse into the nanochannel that changes the hydrophobic properties of nanochannel which depends on the non-covalent interaction between NALC-P5 and –NH group of the R/S-PPL molecule. We further studied the enantioselective transportation of chiral isomers by this chiral NALC-P5 modified gold electrode, the strategy shown in Scheme 1. Firstly, chiral NALC-P5 was adjusted to the nanochannel internal surface of the PC membrane and this modified PC membrane was fabricated on the surface of the gold electrode which was used as the working electrode (denoted as "Chiral sensor" in Scheme 1). Differential pulse voltammetry (DPV) and electrochemical impedance spectroscopy (EIS) was employed to quantify chiral drug PPL isomers based on the transmission of electroactive ions and obtained current signals from the two electrode.

|
Download:
|
| Scheme 1. Schematic drawing for chiral recognition of PPL enantiomer using nanochannel modified electrochemical sensor. | |
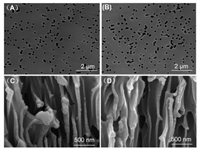
The porous nanochannel membranes were obtained with pre-etched nanopores with different size available to make sure the nanopore was coherent. The morphology and microstructure of nanochannel before and after modification were investigated by SEM. Fig. 1A shows the size of the nanopore was about 100 nm. The surface of the etched film has uniformly distributed nanochannels, and the pore size does not change significantly before and after modification which indicates that the modification process has negligible effect on the size of the channel and the morphology of the surface of the film. In Fig. 1C, the straight pore structure of the nanochannel can be seen in the cross-sectional view of the film that suggests the attachment a PC film with nanochannels. To confirm the successful functionalization of NALC-P5 into the inner surface of the membrane, we performed XPS experiment of the membrane before and after modified with NALC-P5 and compared the experimental results (chemical states and composition analysis, Fig. S4 in Supporting information). The existence of S element in the XPS spectrum of the NALC-P5 modified membrane is indicated the presence of NALC-P5 on the inner surface of the membrane surface.
|
Download:
|
| Fig. 1. The SEM images for the PC nanochannel. (A) The surface morphology of the nanochannel before and (B) after modified with NALC-P5. (C) The cross sction of the nanochannel before and (D) after modified with NALC-P5. | |
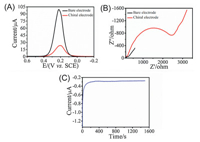
Initially, we tested the electrochemical performance of the prepared chiral sensing electrode and compared with a clean gold disk electrode, 3 mL of 5 mmol/L K3[Fe(CN)6]/K4[Fe(CN)6] solution as an electrolyte added to the electrolytic cell, and the prepared chiral sensing electrode used as the working electrode. Differential pulse voltammetry (DPV), electrochemical impedance spectroscopy (EIS) and current-time tests were performed respectively. The blank gold disk electrode was also tested for DPV and EIS. It was observed from Fig. 2 that after addition of the modified PC film to the electrode surface, the DPV signal was only about 10% of the blank electrode, and the resistance value was increased from about 200 Ω to 2500 Ω, indicating that the PC film with nanochannels was successfully combined to the electrode surface to achieve the transmission of electroactive ions, and the stability of the current-time signal indicates that the prepared working electrode is very stable.
|
Download:
|
| Fig. 2. Electrochemical characterization before and after preparation of chiral sensing electrodes: (A) DPV images, (B) EIS images, (C) Current-time images. | |
Primarily the electrode was immersed in the PPL solution, and to verify the critical relationship between immersion time and the electrochemical signal, the cyclic voltammetry (CV) experiment was performed in S-PPL solution with different time interval. The experimental results were shown in Fig. S5A (Supporting information). The CV signal is stable after soaking for 10 min and as time continued, the signal changes were very small, so in the subsequent experiment, the immersion time was determined to be 10 min. We further investigated the concentration-response of the prepared chiral sensor electrodes to chiral drug PPL, we first prepared 1 mmol/L R-PPL and S-PPL aqueous solutions, and then dilute this solution layer-by-layer to acquire PPL with the concentrations of 10-4 mol/L, 10-5 mol/L, 10-6 mol/L, 10-7 mol/L, 10-8 mol/L and 10-9 mol/L. The modified PC film was cut into two identical parts to make a chiral sensing electrode and used 5 mmol/L K3[Fe(CN)6]/K4[Fe(CN)6] as the electrolyte for DPV experiment. In the beginning, the concentration of the chiral drug is 0, and the measured current signals of the two electrodes are close, indicating the consistency of the initial state of the two electrodes. Then the electrode was immersed in R/S-PPL solution, and the DPV signal at 0.21 V was measured. The results are shown in Fig. S5B (Supporting information) and it was observed that after soaked in PPL solution, all the current signal of the electrode attenuated, but compare to R-PPL the signal immersed in the S-PPL at the same concentration attenuated more. Based on the above results, we concluded that at higher concentrations of S-PPL more interaction occurred between –NH group of the S-PPL and NALC-P5. Simultaneously, the hydrophobic nature of S-PPL containing nanochannel increase consequently that prevent the movement of electrolyte. Due to this reason, the prepared chiral sensor electrode can produce a differentiated electrochemical response signal to the R/S-PPL isomers and the higher the PPL concentration is, the more noticeable this difference will be. To maintain a worthy electrochemical response, in subsequent experiments, we used a concentration of 1 mmol/L chiral drug R/S-PPL isomers for experiments.
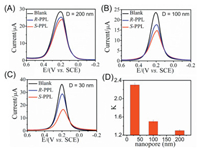
The changes of current in this experiment reflect the change in the concentration of electroactive ions on the surface of the electrode, these changes due to the transmission of electroactive ions across the nanochannel. To pursue more prominent recognition performance, it is necessary to study the factors that can affect the ion transport through the nanochannel to obtain the optimal result, and the pore size of the nanochannel is a key factor, this size directly affects the chiral recognition performance of the channel. We investigated the importance of the nanochannel size in the chiral recognition performance. Therefore we further compare the discrimination performance of PPL with chiral sensing electrodes using nanochannels on the PC membrane as recognition sites and firstly prepared chiral sensing electrode using modified 200 nm pore size PC membranes, the electrodes were soaked in 1 mmol/L R-PPL, S-PPL and the same solution without PPL for 10 min, and then their DPV signals were measured in K3[Fe(CN)6]/K4[Fe(CN)6] electrolyte using an electrochemical workstation. The changes of current at about 0.21 V under the addition of PPL are shown in Fig. 3A. Compared with the current without PPL, the current under the addition of PPL was reduced, and for the addition of S-PPL, the current signal attenuated more. To quantitatively illustrate the chiral recognition ability of the electrode, we determine the change ratio of the peak current K in the DPV signals under the same concentration of enantiomers, which was defined in Supporting information (we obtained the chiral sensing electrodes combined with PC membrane with a channel diameter of 200 nm according to the same preparation and modification methods, and its K value is about 1.3).
|
Download:
|
| Fig. 3. Chiral recognition of PPL enantiomer in different diameter of the nanochannel: (A) 200 nm, (B) 100 nm, (C) 30 nm. (D) The recognition ability (K) of the electrode toward PPL with different diameters of the nanochannel. | |
Another important aspect is the pore diameter in the nanochannel of this chiral sensing platform which directly affects the current signal and chiral recognition performance. Following the 200 nm PC membrane, we continue to investigate the effect of reduction in nanochannel pore diameter for the chiral discrimination performance. Using the same preparation and modification methods, we coated the electrode with the nanochannel pore diameter of 100 nm. The experimental steps were same as 200 nm PC membrane coated electrode and the result is shown in Fig. 3B, the difference in current signal between R-PPL and S-PPL was higher than 200 nm nanochannel. Finally, when the diameter of the nanochannel was reduced to 30 nm, as shown in Fig. 3C, the largest current difference was observed between PPL isomers. Similarly, we have calculated K value of various diameter (200 nm, 100 nm, 30 nm) of nanochannel which is shown in Fig. 3D. The K value increases with decreasing the pore diameter of nanochannel, and the highest K value is calculated for 30 nm nanochannel which is 2.3, this result strongly indicating that the chiral recognition performance of the electrode was much improved with decreasing of pore diameter of the nanochannel.
The above results concluded that in a smaller pore diameter nanochannel chiral NALC-P5/PPL groups are close to each other which increase the hydrophobicity of nanochannel that direct effects on the transmission of electroactive ions and electrochemical signal. Additionally, to further illustrate the important role of chiral NALC-P5 in electrode chiral recognition performance, we fabricated a new electrode with 30 nm nanochannel which does not modify with NALC-P5 and other conditions remain the same. The electrodes were immersed in the PPL enantiomer at the same concentration for 10 min, and then analyzed by DPV and EIS in 5 mmol/L K3[Fe(CN)6]/K4[Fe(CN)6] electrolyte. The response signals generated by the electrode were shown in Fig. S6 (Supporting information). The current signals generated by the electrode which immersed in PPL isomer solutions is the same, and it is close to the signal generated by the electrode soaked in the non-PPL solution, both the DPV and EIS results indicating that the electrode without NALC-P5 not having the ability to distinguish the chiral drugs R/S-PPL whereas the nanochannel modified with chiral NALC-P5 can effectively distinguish R/S-PPL isomers.
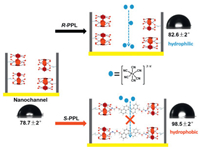
The above mention experimental results established that the chiral NALC-P5 modified nanochannels can differentiate chiral drug R/S-PPL and can output the identification signal as an electrochemical signal. The reduction of the pore size of the nanochannel in the presence of chiral NALC-P5 helps to further improve the electrode chiral recognition performance and produce a better recognition effect. Further, we investigated the recognition mechanism and physical properties changes of the NALC-P5 modified nanochannels after interaction with chiral drug R/S-PPL through a contact angle experiment. In this experiment, we measured the contact angle of the NALC-P5 modified nanochannels before and after immersion in R/S-PPL solution for 10 min. The changes of the contact angle of the membrane using water droplets, as shown in Fig. 4, both the contact angles was increased when soaked in PPL isomer solutions, and after soaked in S-PPL, the membrane became hydrophobic. The above results show that there is an interesting mechanism for chiral R/S-PPL identification within the nanochannel. When the electrode was soaked in PPL solution, PPL molecules can diffuse into the nanochannel and generate host-guest recognition with NALC-P5, the recognition configuration depends on the non-covalent interaction between –NH group of the PPL molecule and NALC-P5. While the naphthalene ring of PPL is outside the Pillar[5]arene due to its large size, for a pair of isomers, the interaction with NALC-P5 was quite different, so the hydrophobicity of the nanochannel inner surface was different.
|
Download:
|
| Fig. 4. The contact angle results and possible mechanism of the chiral sensor. (Soaked time 10 min, concentration of R/S-PPL solution is 1 mmol/L, and pore diameter of nanochannel is 30 nm). | |
Additionally, to further illustrate the concept of recognition of R/S-PPL, we investigated the interaction between NALC-P5 and PPL isomers through 1H NMR spectrum. 1H NMR spectra of R/S-PPL, NALC-P5 and an equimolar mixture of R/S-PPL with NALC-P5 were measured separately at the concentration of 1 mmol/L in DMSO-d6 at 298 K which shown in Fig. S7 (Supporting information). Upon addition of NALC-P5 against R-PPL, the protons on the chiral carbon of the R-PPL were shifted upfield by 0.019 ppm whereas the proton of host molecules was shifted downfield by 0.011 ppm. On the other hand, the 1H NMR spectrum for S-PPL shows strong chemical shifts, the protons on the chiral carbon of the S-PPL were shifted upfield by 0.027 ppm and the proton of host molecules were shifted downfield by 0.020 ppm that indicated the host-guest interaction of R-PPL and S-PPL towards NALC-P5 is different phenomena.
To established the nature of chemical shift fitting, binding mode, and association constant (Ka) of NALC-P5 towards R-PPL and S-PPL 1H NMR titration experiments are shown in Figs. S8 and S9 (Supporting information), respectively. From Fig. S8, it is suggested that 1H NMR signals correspond to chiral carbon atom shifted considerably with the increasing of R-PPL concentration and 1.0 equiv. of R-PPL resulted in a maximum downfield shift. Additionally, negligible chemical shifting is observed over 1.0 equiv. concentration of a guest molecule, similar phenomena observed for S-PPL, which indicates the 1:1 stoichiometry between NALC-P5 and R/S-PPL. The Kabetween NALC-P5 and R-PPL was calculated to be 54.1 L/mol using a nonlinear curve fitting analysis, whereas the Ka between NALC-P5 and S-PPL was calculated to be 230.7 L/mol, which suggests that NALC-P5 and S-PPL have a stronger interaction at the molecular level. All experimental details are available in Supporting information.
In conclusion, we demonstrate a simple and sensitive electrochemical method for chiral drug recognition of S/R-PPL isomers through chiral Pillar[5]arene based NALC-P5, PC membrane and gold electrode. We successfully coated NALC-P5 on the various pore diameter constructed nanochannel of PC membrane for better recognition performance and it is observed that the chiral recognition ability of the electrode was much improved with decreasing of pore diameter of the nanochannel. The NALC-P5 and NALC-P5 modified PC membrane are characterized by 1H NMR and SEM experiments respectively. The 1:1 host-guest interaction between NALC-P5 and R/S-PPL isomers were confirmed by 1H NMR titration, also recognition mechanism between NALC-P5 and R/S-PPL established through contact angle measurements which depend on the changes in the hydrophobicity of the nanochannels. This method not only provides a reference for the development of a new type of chiral electrochemical sensor but also shows great potential in the identification and separation of chiral drugs.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was financially supported by the National Key Research and Development Program of China (No. 2018YFD0200102), the National Natural Science Foundation of China (Nos. 21772055, 21572076, 21807083, 21911530178), the Program for Innovative Teams of Outstanding Young and Middle-aged Researchers in the Higher Education Institutions of Hubei Province (No. T201702), the 111 Project (No. B17019E).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2020.11.055.
| [1] |
M. Zhang, G. Qing, T. Sun, Chem. Soc. Rev. 41 (2012) 1972-1984. DOI:10.1039/C1CS15209B |
| [2] |
R. Bentley, Chem. Soc. Rev. 34 (2005) 609-624. DOI:10.1039/b418284g |
| [3] |
M. Liu, L. Zhang, T. Wang, Chem. Rev. 115 (2015) 7304-7397. DOI:10.1021/cr500671p |
| [4] |
A. Berthod, Anal. Chem. 78 (2006) 2093-2099. DOI:10.1021/ac0693823 |
| [5] |
F. Ana, A. Gilberto, F. Amílcar, Biomed. Chromatogr. 28 (2014) 27-58. DOI:10.1002/bmc.3004 |
| [6] |
R. Hazen, D. Sholl, Nat. Mater. 2 (2003) 367-374. DOI:10.1038/nmat879 |
| [7] |
Y. Liu, W. Xuan, Y. Cui, Adv. Mater. 22 (2010) 4112-4135. DOI:10.1002/adma.201000197 |
| [8] |
M. Zhang, B. Ye, Anal. Chem. 83 (2011) 1504-1509. DOI:10.1021/ac102922f |
| [9] |
S. Tang, Q. Bin, W. Chen, et al., J. Chromatogr. A 1440 (2016) 112-122. DOI:10.1016/j.chroma.2016.02.053 |
| [10] |
M. Okamoto, J. Pharmaceut. Biomed. 27 (2002) 401-407. DOI:10.1016/S0731-7085(01)00646-X |
| [11] |
C. Yamamoto, Y. Okamoto, Bull. Chem. Soc. Jpn. 77 (2004) 227-257. DOI:10.1246/bcsj.77.227 |
| [12] |
J. Zhang, S. Xie, M. Zhang, et al., Anal. Chem. 86 (2014) 9595-9602. DOI:10.1021/ac502073g |
| [13] |
J. He, X. Wang, M. Morill, S.A. Shamsi, Anal. Chem. 84 (2012) 5236-5242. DOI:10.1021/ac300944z |
| [14] |
G. Gubitz, M. Schmid, Electrophoresis 25 (2004) 3981-3996. DOI:10.1002/elps.200406173 |
| [15] |
I. Boussouar, Q. Chen, X. Chen, et al., Anal. Chem. 89 (2016) 1110-1116. DOI:10.1021/acs.analchem.6b02682 |
| [16] |
J. Zhang, Z. Li, W. Gong, et al., Inorg. Chem. 55 (2016) 7229-7232. DOI:10.1021/acs.inorgchem.6b00894 |
| [17] |
X. Hou, W. Guo, L. Jiang, Chem. Soc. Rev. 40 (2011) 2385-2401. DOI:10.1039/c0cs00053a |
| [18] |
C. Yuan, X. Wu, R. Gao, et al., J. Am. Chem. Soc. 141 (2019) 20187-20197. DOI:10.1021/jacs.9b10007 |
| [19] |
J. Li, D. Stein, C. McMullan, et al., Nature 412 (2001) 166-169. DOI:10.1038/35084037 |
| [20] |
C. Ho, R. Qiao, J. Heng, et al., Proc. Natl. Acad. Sci. U. S. A. 102 (2005) 10445-10450. DOI:10.1073/pnas.0500796102 |
| [21] |
S. Wu, S. Park, X. Ling, Nano Lett. 6 (2006) 2571-2576. DOI:10.1021/nl0619498 |
| [22] |
D. Zhang, Q. Wang, X. Fan, et al., Adv. Mater. 30 (2018) e1804862. DOI:10.1002/adma.201804862 |
| [23] |
L. Wen, Z. Sun, C. Han, et al., Chem. Eur. J. 19 (2013) 7686-7690. DOI:10.1002/chem.201300528 |
| [24] |
Y. Zhu, K. Zhan, X. Hou, ACS Nano 12 (2018) 908-911. DOI:10.1021/acsnano.7b07923 |
| [25] |
M. Wang, H. Meng, D. Wang, et al., Adv. Func. Mater. 31 (2019) 1805130. DOI:10.1002/adma.201805130 |
| [26] |
K. Zhan, Z. Li, J. Chen, et al., Nano Today 33 (2020) 100868. DOI:10.1016/j.nantod.2020.100868 |
| [27] |
R. Wang, Y. Sun, F. Zhang, et al., Angew. Chem. Int. Ed. 56 (2017) 5294-5298. DOI:10.1002/anie.201702175 |
| [28] |
Y. Sun, J. Ma, F. Zhang, et al., Nat. Commun. 8 (2017) 160-165. DOI:10.1038/s41467-017-00233-z |
| [29] |
F. Zhang, J. Ma, Y. Sun, et al., Anal. Chem. 90 (2018) 8270-8275. DOI:10.1021/acs.analchem.8b01948 |
| [30] |
F. Zhang, J. Ma, Y. Sun, et al., Chem. Sci. 7 (2016) 3227-3233. DOI:10.1039/C5SC04726A |
| [31] |
Y. Sun, F. Zhang, J. Quan, et al., Nat. Commun. 9 (2018) 2617-2624. DOI:10.1038/s41467-018-05103-w |
| [32] |
F. Zhang, Y. Sun, D. Tian, H. Li, Angew. Chem. Int. Ed. 56 (2017) 7186-7190. DOI:10.1002/anie.201701255 |
| [33] |
S. Lu, Z. Guo, Y. Xiang, L. Jiang, Adv. Mater. 28 (2016) 9851-9856. DOI:10.1002/adma.201603809 |
| [34] |
G. Xie, P. Li, Z. Zhao, et al., Angew. Chem. Int. Ed. 57 (2018) 16708-16712. DOI:10.1002/anie.201809627 |
| [35] |
L. Wen, Z. Sun, C. Han, et al., Chem. Eur. J. 19 (2013) 7686-7690. DOI:10.1002/chem.201300528 |
| [36] |
C. Han, X. Hou, H. Zhang, et al., J. Am. Chem. Soc. 133 (2011) 7644-7647. DOI:10.1021/ja2004939 |
| [37] |
S. Zhang, I. Boussouar, H. Li, Chin. Chem. Lett. (2020), doi: http://dx.doi.org/10.1016/j.cclet.2020.06.035.
|
| [38] |
R.H. Li, J. Ma, Y. Sun, H. Li, Chin. Chem. Lett. 31 (2020) 3095-3101. DOI:10.1016/j.cclet.2020.06.041 |
| [39] |
P. Li, Y. Chen, Y. Liu, Chin. Chem. Lett. 30 (2019) 1190-1197. DOI:10.1016/j.cclet.2019.03.035 |
| [40] |
Z. Liu, J. Wu, C. Wang, et al., Chin. Chem. Lett. 30 (2019) 2299-2303. DOI:10.1016/j.cclet.2019.10.023 |
| [41] |
T. Xiao, L. Zhou, X.Q. Sun, et al., Chin. Chem. Lett. 31 (2020) 1-9. DOI:10.1016/j.cclet.2019.05.011 |
| [42] |
T. Ogoshi, S. Kanai, S. Fujinami, et al., J. Am. Chem. Soc. 130 (2008) 5022-5023. DOI:10.1021/ja711260m |
| [43] |
X.Y. Lou, Y.W. Yang, Adv. Mater. 32 (2020) 2003263. DOI:10.1002/adma.202003263 |
| [44] |
N. Song, X.Y. Lou, L. Ma, et al., Theranostics 9 (2019) 3075-3093. DOI:10.7150/thno.31858 |
| [45] |
N. Song, T. Kakuta, T. aki Yamagishi, et al., Chem 4 (2018) 2029-2053. DOI:10.1016/j.chempr.2018.05.015 |
| [46] |
N. Song, X.Y. Lou, H. Yu, et al., Mater. Chem. Front. 4 (2020) 950-956. DOI:10.1039/C9QM00741E |
| [47] |
X.S. Li, Y.F. Li, J.R. Wu, et al., J. Mater. Chem. A 8 (2020) 3651-3657. DOI:10.1039/C9TA13776A |
| [48] |
M. Xue, Y. Yang, X. Chi, Z. Zhang, F. Huang, Acc. Chem. Res. 45 (2012) 1294-1308. DOI:10.1021/ar2003418 |
| [49] |
L. Luo, G. Nie, D. Tian, et al., Angew. Chem. Int. Ed. 55 (2016) 12713-12716. DOI:10.1002/anie.201603906 |
| [50] |
L. Tan, H. Li, Y. Zhou, et al., Small 11 (2015) 3807-3813. DOI:10.1002/smll.201500155 |
 2021, Vol. 32
2021, Vol. 32 

