Radiotherapy is one of the most commonly used methods in tumor treatment, but the efficacy is greatly influenced due to the dose-limiting of radiotherapy and drug toxicity [1-3]. A good radiosensitizer (RS) at least needs to have two characteristics high efficiency and low toxicity. The search for efficient radiosensitizer for radiotherapy with low-toxicity continues to be a challenge to be solved in clinic. Although the research of RS has made some progress in recent years, the efficiency and selectivity of RS still needs to be further improved [4, 5]. Among the various radiosensitizers, high-Z (high atomic number) based coordination complexes have attracted much attention in recent years since they can improve photoelectric effect and Compton effect to enhance the efficiency and specificity of radiotherapy [6-9]. Besides the well-established platinum-based (Z=78) complexes [10, 11], iridium (Z=77) [12, 13] and ruthenium (Z=44) [14-16] complexes have also been reported as potential RS candidates. On the other hands, osmium (Z=76) with similar Z value of platinum and belong to the same sub-group of ruthenium, osmium complexes should be one of the most promising candidates. To the best of our knowledge, osmium-based complexes using as RS have not been reported until now [17]. Herein we report the first osmium-based coordination complex RS, OsN(PhenOH)Cl3 (PhenOH=1, 10-phenanthrolin-2-ol), a high oxidation state osmium(Ⅵ) nitrido complex using as highly X-ray sensitive low-toxicity radiotherapeutic drug (Fig. 1a).
|
Download:
|
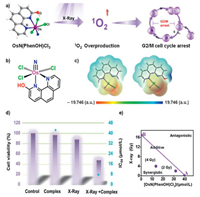
| Fig. 1. (a) Highly X-ray sensitive low-toxicity radiotherapeutic Os(Ⅵ) nitrido complex induced G2/M cell cycle arrest. (b) Structure of OsN(PhenOH)Cl3. (c) MESP plots of OsN(Phen)Cl3 and OsN(PhenOH)Cl3. The atoms in red, gray, blue, green and white denote oxygen, carbon, nitrogen, chlorine and hydrogen, respectively. (d) Cytotoxic effects of OsN(PhenOH)Cl3 (2.5 μmol/L) with/without radiation of X-ray (4 Gy) on Hela cells. (e) Isobologram analysis of the synergistic antiproliferative effect of the combined treatment of X-ray and OsN(PhenOH)Cl3 on Hela cells. The data points in the isobologram correspond to the growth inhibition ratio at 50% in the combined treatment. | |
Recently, Lau [18-21] and Lippard [22-24] reported that osmium(Ⅵ) nitrido was a useful platform for the construction of molecular metal-based anti-cancer drugs. Osmium(Ⅵ) nitrido complexes bearing organic ligands exhibit excellent anticancer properties, and slightly ligand changes can effectively regulate their anticancer activity. Of all the reported osmium(Ⅵ) nitrido anticancer drugs (Table S1 in Supporting information), OsN(Phen)Cl3 (Phen=1, 10-phenanthroline) has been studied most detailly [22-24]. It can cause DNA damage by breaking Os-Cl bond to release chloride ligand, thus inducing apoptosis of various cancer cells and cancer stem cells. Based on these reasons, OsN(PhenOH)Cl3 (Fig. 1b) was designed as a highly efficient RS with low toxicity.
Introducing strong electron-donating substituent to ligand to enhance Os-Cl bond for reducing chloride release is a desired pathway to lower down the toxicity of OsN(Phen)Cl3. In this work, −OH group was chosen as the representative of strong electron-donating substituent and the designed OsN(PhenOH)Cl3 was synthesized and fully characterized (Figs. S1 and S8 in Supporting information). From the calculated results we can see, the bond length (Table S2 in Supporting information) of Os-Cl and Os≡N in OsN(PhenOH)Cl3 is obviously shorter than that in OsN(Phen)Cl3, and the electron density (Fig. 1c) of Os-Cl and Os≡N in OsN(PhenOH)Cl3 is significantly higher than that in OsN(Phen)Cl3, which elucidated that the introduction of −OH group can regulate the coordination environment of osmium(Ⅵ) nitrido complex.
In order to check the regulatory effect of ligand substitution on cytotoxicity, MTT assay was employed against human cervical cancer (Caski and HeLa) and normal (E6E7/Ect) cell lines to further verification. From the data presented in Table S3 (Supporting information), we can see OsN(Phen)Cl3 shown strong toxicity to human cervical cancer cell lines (IC50: 8.40 μmol/L for Caski and 2.40 μmol/L for HeLa) and normal cervical cell lines (IC50: 6.89 μmol/L), while OsN(PhenOH)Cl3 shown much lower toxicity to cell lines (IC50: 32.97 μmol/L for Caski and 42.70 μmol/L for HeLa), especially to normal cells (IC50: 52.03 μmol/L). Obviously, the change of cytotoxicity is due to the introduction of −OH group, the enhancement of Os-Cl bond is the major reason. On the other hand, the −OH group introduction also decreased the lipophilicity of the complex (Fig. S2a in Supporting information), which leads to the decrease of cell absorption (Fig. S2b in Supporting information) and thus also a possible reason for the lower cytotoxicity (Table S3). These results clearly proved the substituent regulation on cytotoxicity and illustrated OsN(PhenOH)Cl3 is a low toxic drug.
To investigate the RS efficacy of OsN(PhenOH)Cl3, we checked its X-ray sensitivity. Fig. 1d shown the cell viability rate of HeLa cells treated with OsN(PhenOH)Cl3 (2.5 μmol/L) and/or X-ray for 72 h. As shown in Fig. 1d, the cell viability of single Os nitrido drug or X-ray treatment was similar to that of the blank control group, and the cell viability decreased significantly after Os nitrido drug and X-ray treatment. At the same time, after the co-treatment of Os drug and X-ray, the IC50 value decreased from 42.70 μmol/L to 8.17 μmol/L, which means that the anticancer efficacy increased more than 500% (sensitivity enhancement ratio: 5.23) (Fig. 1d and Fig. S7 in Supporting information). Furthermore, the radiosensitization property of the Os nitrido complex was identified by equivalent line analysis (Fig. 1e), and the stability of the complex under irradiation was verified by UV–vis spectra (Fig. S3 in Supporting information).
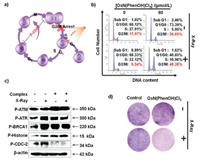
The highly sensitivity to X-ray prompted us to further study the action mechanism of the Os nitrido drug. We used flow cytometry analysis to confirm the cell cycle conditions during the treatment represented by HeLa cell lines (Fig. 2a). The results were presented in Fig. 2b and Fig. S4 (Supporting information). From the figures we can see, the peak of G2/M phase in the control group was 11.97%, which increased to 20.65% after treatment with Os nitrido drug, and further increased to 48.38% after the X-ray combined treatment. This indicated that the co-treatment can induced G2/M cell cycle arrest. This was further improved by western blotting experiment (Fig. 2c). Through western we found that the phosphorylation of CDC-2, a protein that regulates the entry of cells into mitosis [25], expression was significantly inhibited. On the other hand, the phosphorylation of DNA repair related kinases, such as ATM (ataxia telangiectasi amutated kinase)/ATR (ataxia telangiectasia kinase)/BRCA1 protein, increased rapidly. Furthermore, the phosphorylation of histone, a protein highly related to DNA expression, was also observed. These phenomena together prove the fact that radiotherapy sensitized by Os(Ⅵ) nitrido complex leads to DNA damage and eventually causes G2/M cell cycle arrest. The colony formation assay further confirmed the inhibition effect on the proliferation of HeLa cells. As can be seen from Fig. 2d, the cell proliferation decreased seriously after drug and X-ray therapy.
|
Download:
|
| Fig. 2. Anti-tumor mechanism of co-treatment of Os(N)(PhenOH)Cl3 and X-ray irradiation. (a) Schematic diagram of G2/M cell cycle arrest in Hela cells induced by OsN(PhenOH)Cl3 combined with irradiation. (b) Flow cytometry analysis of the effect combined treatment of X-ray (4 Gy) and OsN(PhenOH)Cl3 (60 μmol/L) in the cell cycle distribution of Hela cells. (c) Western blot analysis for the expression of P-ATM, P-ATR, P-BRCA1, P-histone and P-CDC-2. β-Actin was used as loading control. The concentration of OsN(PhenOH)Cl3 was 60 μmol/L and the dose of radiation was 4 Gy. (d) Representative photographs of colony formation of Hela cells under the co-treatment of OsN(PhenOH)Cl3 (8 μmol/L) and X-ray irradiation (4 Gy) for 8 days. | |
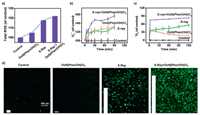
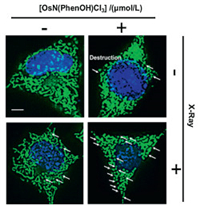
In order to further understand the cause of G2/M cell cycle arrest, we checked the changes in intracellular ROS level, since ROS was reported to be the primary mediator of apoptosis [26]. As can be seen from Figs. 3a and d, the intracellular ROS (reactive oxygen species) level increased significantly after the Os(Ⅵ) nitrido drug and X-ray combination treatment by employing DCFH-DA (dichloro-dihydro-fluorescein diacetate) probe [27], the most widely used probe for intercellular ROS. Among various kinds of ROS (Figs. 3b and c, Table S4 and Fig. S5 in Supporting information), the increase of superoxide radical (detected by DHE (dihydroethidium) probe [28, 29]) and singlet oxygen species (detected by DPBF (1, 3-diphenylisobenzofuran) probe [30, 31]) were particularly significant. As ROS is mainly produced in mitochondria, the increase of ROS will cause mitochondrial damage and accompanied with mitochondrial dysfunction. This was also observed by MitoTracker labelling and Mitochondrial Membrane Potential experiments [32-34]. As shown in Fig. 4 and Fig. S6 (Supporting information), the mitochondria of Os(Ⅵ) nitrido drug and X-ray treated cells changed from filamentous to punctate and significant depletion of ΔΨm was detected. These results indicated that Os(Ⅵ) nitrido drug and X-ray treatment induces G2/M cell cycle arrest by increasing intracellular ROS to mediate the mitochondrial pathway and DNA damage.
|
Download:
|
| Fig. 3. Examination of ROS level of Hela cells combined treatment of X-ray (4 Gy) and OsN(PhenOH)Cl3 (15 μmol/L) by using (a) DCFH-DA probe, (b) DHE probe and (c) DPBF probe. (d) Fluorescence picture of the effect of ROS in Hela cells by using DCFH-DA probe. Scale bar = 400 μm. | |
|
Download:
|
| Fig. 4. Morphological changes of mitochondria due to combined treatment of X-ray (4 Gy) and/or OsN(PhenOH)Cl3 (15 μmol/L) in Hela cells. The arrow represents mitochondrial fragmentation. Scale bar =10 μm. | |
In summary, we designed and synthesized a high efficiency and low toxicity radiosensitizer, OsN(PhenOH)Cl3, through substituent regulation. To the best of our knowlege, this is the first osmium-based coordination complex radiosensitizer. The experimental results showed that this radiosensitizer induced G2/M cell cycle arrest mainly through increasing intracellular ROS. Overall, this study provides information for future rational design high efficiency and low toxicity radiosensitizer based-on metal coordination complexes for selective cancer therapy.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work was supported by the Natural Science Foundation of China (Nos. 21701054, 21877049), Guangdong Natural Science Foundation (No. 2017A030310409), the Open Fund of the Key Laboratory of Functional Molecular Engineering of Guangdong Province (No. 2017KF06), Major Program for Tackling Key Problems of Industrial Technology in Guangzhou (No. 201902020013), Pearl River Nova Program of Guangzhou (No. 201906010077), Dedicated Fund for Promoting High-Quality Marine Economic Development in Guangdong Province (Nos. GDOE-2019-A31, 2020-035), Fundamental Research Funds for the Central Universities, Guangdong Provincial Key Laboratory Fund of Functional Supramolecular Coordination Materials and Applications and K. C. Wong Education Foundation.
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.11.050.
| [1] |
A.C. Begg, F.A. Stewart, C. Vens, Nat. Rev. Cancer 11 (2011) 239-253. DOI:10.1038/nrc3007 |
| [2] |
J.F. Bosset, L. Collette, G. Calais, et al., N. Engl. J. Med. 355 (2006) 1114-1123. DOI:10.1056/NEJMoa060829 |
| [3] |
Y. Chang, L. He, Z. Li, et al., ACS Nano 11 (2017) 4848-4858. DOI:10.1021/acsnano.7b01346 |
| [4] |
J.D. Cox, J. Stetz, T.F. Pajak, Int. J. Radiat. Oncol. Biol. Phys. 31 (1995) 1341-1346. DOI:10.1016/0360-3016(95)00060-C |
| [5] |
T.L. Chevalier, R. Arriagada, E. Quoix, et al., J. Natl. Cancer Inst. 83 (1991) 417-423. DOI:10.1093/jnci/83.6.417 |
| [6] |
G. Song, Y. Chen, C. Liang, et al., Adv. Mater. 28 (2016) 7143-7148. DOI:10.1002/adma.201602111 |
| [7] |
T.T. Jia, G. Yang, S.J. Mo, et al., ACS Nano 13 (2019) 8320-8328. DOI:10.1021/acsnano.9b03767 |
| [8] |
Z. Zhao, P. Gao, L. Ma, T. Chen, Chem. Sci. 11 (2020) 3780-3789. DOI:10.1039/D0SC00862A |
| [9] |
Y. Duo, Y. Huang, W. Liang, et al., Adv. Funct. Mater. 30 (2020) 1906010. DOI:10.1002/adfm.201906010 |
| [10] |
H. Liu, W. Lin, L. He, T. Chen, Biomaterials 226 (2020) 119545. DOI:10.1016/j.biomaterials.2019.119545 |
| [11] |
Y. Yong, C. Zhang, Z. Gu, et al., ACS Nano 11 (2017) 7164-7176. DOI:10.1021/acsnano.7b03037 |
| [12] |
Y. Altundal, G. Cifter, A. Detappe, W. Ngwa, et al., Phys. Med. 31 (2015) 25-30. DOI:10.1016/j.ejmp.2014.11.004 |
| [13] |
S. Corde, J. Balosso, H. Elleaume, et al., Cancer Res. 63 (2003) 3221-3227. DOI:10.1002/cncr.11380 |
| [14] |
J. Liu, H. Lai, Z. Xiong, et al., Chem. Commun. 55 (2019) 9904-9914. DOI:10.1039/C9CC04098F |
| [15] |
L. Wang, T. Zhang, M. Huo, et al., Small 15 (2019) 1903254. DOI:10.1002/smll.201903254 |
| [16] |
Z. Deng, L. Yu, W. Cao, et al., Chem. Commun. 51 (2015) 2637-2640. DOI:10.1039/C4CC07926D |
| [17] |
S.M. MeierMenches, C. Gerner, W. Berger, et al., Chem. Soc. Rev. 47 (2018) 909-928. DOI:10.1039/C7CS00332C |
| [18] |
P. Zhang, H. Huang, Dalton Trans. 47 (2018) 14841-14854. DOI:10.1039/C8DT03432J |
| [19] |
W.X. Ni, W.L. Man, M.T. Cheung, et al., Chem. Commun. 47 (2011) 2140-2142. DOI:10.1039/c0cc04515b |
| [20] |
W.X. Ni, W.L. Man, S.M. Yiu, et al., Chem. Sci. 3 (2012) 1582-1588. DOI:10.1039/c2sc01031c |
| [21] |
Q. Tang, W.X. Ni, C.F. Leung, et al., Chem. Commun. 49 (2013) 9980-9982. DOI:10.1039/c3cc42250j |
| [22] |
K. Suntharalingam, T.C. Johnstone, P.M. Bruno, et al., J. Am. Chem. Soc. 135 (2013) 14060-14063. DOI:10.1021/ja4075375 |
| [23] |
K. Suntharalingam, W. Lin, T.C. Johnstone, et al., J. Am. Chem. Soc. 136 (2014) 14413-14416. DOI:10.1021/ja508808v |
| [24] |
G. Berger, K. Grauwet, H. Zhang, et al., Cancer Lett. 416 (2018) 138-148. DOI:10.1016/j.canlet.2017.11.041 |
| [25] |
J. Chen, L. Li, J. Su, et al., J. Agric. Food Chem. 63 (2015) 6440-6449. DOI:10.1021/acs.jafc.5b01773 |
| [26] |
M. Wang, Y. Wu, C. Xu, et al., Chem. Asian J. 14 (2019) 2648-2655. DOI:10.1002/asia.201900468 |
| [27] |
A. Aranda, L. Sequedo, L. Tolosa, et al., Toxicol. In Vitro 27 (2013) 954-963. DOI:10.1016/j.tiv.2013.01.016 |
| [28] |
Y. Yang, Y. Wang, L. Xu, T. Chen, Chin. Chem. Lett. (2020), doi: http://dx.doi.org/10.1016/j.cclet.2020.10.012.
|
| [29] |
P. Gao, C. Mei, L. He, et al., Drug Deliv. 25 (2018) 1811-1825. DOI:10.1080/10717544.2018.1494224 |
| [30] |
R. Wu, H. Wang, L. Hai, et al., Chin. Chem. Lett. 31 (2020) 189-192. DOI:10.1016/j.cclet.2019.05.004 |
| [31] |
K. Wang, W. Ma, Y. Xu, X. Liu, et al., Chin. Chem. Lett. 31 (2020) 3149-3152. DOI:10.1016/j.cclet.2020.08.039 |
| [32] |
K. Hayakawa, E. Esposito, X. Wang, et al., Nature 535 (2016) 551-555. DOI:10.1038/nature18928 |
| [33] |
Z.Y. Pan, C.P. Tan, L.S. Rao, et al., Angew. Chem. Int. Ed. 59 (2020) 18755-18762. DOI:10.1002/anie.202008624 |
| [34] |
H. Chao, S. Kuang, L. Sun, et al., Angew. Chem. Int. Ed. 59 (2020) 20697-20703. DOI:10.1002/anie.202009888 |
 2021, Vol. 32
2021, Vol. 32 

