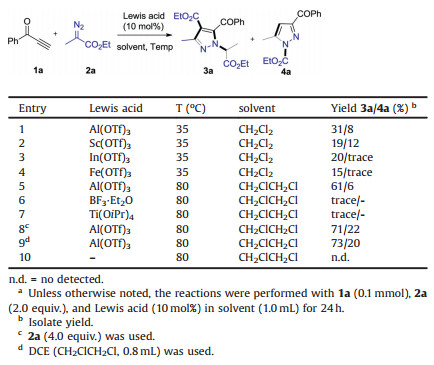
Pyrazole-containing derivatives have exhibited promising biological activities [1], and several newly approved small molecule drugs, such as elexacaftor, erdafitinib, cilastatin, remogliflozin etabonate, bear this kind of two nitrogen atoms-based aromatic heterocycle (Scheme 1a) [2]. A fruitful synthetic approach to substituted pyrazoles has been disclosed during the past two decades (Scheme 1b) [3]. These methods included the construction of two C-N bonds via condensation of 1, 3-dicarbonyl compounds or ynones with hydrazines (path ⅰ) [4], simultaneous formation of one C-N bond and one C-C bond via [3 + 2] cycloaddition of 1, 3-dipoles (path ⅱ), i.e., hydrazones or α-diazo compounds with alkynes or alkenes-bearing electron-withdrawing groups. In addition, the direct N-N bond formation to install into multisubstituted pyrazoles from nitriles via metal-imido intermediates was also available (path ⅲ) [5]. An example of forming one C-N bond via intramolecular cyclization of vinyldiazoacetates was discovered recently (path ⅳ) [6].
|
Download:
|
| Scheme 1. Representative pyrazole-containing drugs and general methods for the construction of pyrazole rings. | |
α-Diazoesters compounds are prominent building blocks for organic synthesis [7]. Cycloaddition reactions of diazo compounds with dipolarophiles bearing carbon-carbon double or triple bond to convert into pyrazolines and pyrazoles derivatives (Scheme 1b, path ⅱ) have received considerable interest [8]. One elegant example was InCl3 catalyzed cycloaddition between diazocarbonyl compounds to alkynes in water initiated by Li's group. When α-diazoarylacetate was used as the 1, 3-dipole to react with methyl propiolate, 4-aryl-3, 5-diester substituted pyrazole was isolated as the major product. This cycloaddition-migration cascade rendered the convenient introduction of substituted group into pyrazolering. As an ongoing research about the reactions of α-diazo carbonyl compounds in the presence of Lewis acids or transitionstate metal catalysts [9], we found that Al(OTf)3 could promote the formation of four-substituted pyrazoles from alkyl α-diazoacetates and ynones. The reaction was found to proceed by a [3 + 2] cycloaddition, 1, 5-ester shift and 1, 3-H shift, then a N-H insertion cascade. Herein, we wish to report the one-pot synthesis of N-alkyl 3-alkyl-4-ester-5-benzoyl substituted pyrazoles.
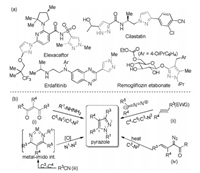
We initiated our study by selection proper catalysts for the reaction between alkynone 1a with ethyl 2-diazopropanoate 2a. It was found that the four substituted pyrazole 3a was detected as the major product, along with three-substituted pyrazole 4a and other unidentified byproducts (for details see the Supporting information). In the presence of 10 mol% of Al(OTf)3 at 35 ℃, the product 3a was obtained in 31% yield and 4a in 8% yield (Table 1, entry 1). Stronger Lewis acids, such as Sc(OTf)3, In(OTf)3 and Fe(OTf)3 could afforded the pyrazole 3a with low yields (entries 2–4), but the reaction in the presence of other metal salts was messy. The yield of the product 3a increased to 61% at 80 ℃ in DCE (entry 5). At this reaction temperature, only trace product 3a can be detected when BF3·Et2O or Ti(OiPr)4 was selected as the catalyst (entries 6 and 7). A better yield can be achieved by increasing the amount of diazoester 2a (entry 8). Furthermore, when reducing the amount of solvent, the pyrazole 3a could be isolated in a yield of 73%, as well as the pyrazole 4a in 20% yield (entry 9). It was noteworthy that the reaction did not occur without a Lewis acid catalyst at high reaction temperature (entry 10).
|
|
Table 1 Optimization of reaction conditions.a |
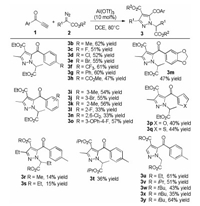
With the optimized reaction condition in hand, we turned to explore the substrate scope of the catalytic system (Scheme 2). A wide range of terminal alkynones with benzoyl group bearing both electron-withdrawing and electron-donating substituents at the para-, meta- or ortho-positions, all reacted smoothly with ethyl 2-diazopropanoate 2a to form the corresponding pyrazole products 3b-3o in moderate yield. It was found that the methyl-substituent at para-position was better than ortho- and meta-positions. The structure of the product 3f was confirmed by single-crystal X-ray diffraction analysis. The supplementary crystallographic data of 3f (CCDC: 1984363) can be obtained free of charge from The Cambridge Crystallographic Data Centre. Functional substituents such as halogens, alkyl groups and esters were well tolerated. Furthermore, ynones bearing 3-furyl or 3-thiophyl group also proved to be suitable substrates, affording 3p in 40% yield and 3q in 44% yield. Subsequently, the substrate scope with respect to the α-diazoesters was examined. The length of α-alkyl chain attached to α-diazoesters had a huge effect on the yield (3r and 3s), and the corresponding NH-pyrazole was isolated as the major product (53% yield) when methyl 2-diazobutanonate was used (see Supporting information for details). Next, by changing the esters group of diazopropanoate from methyl to isopropyl group, the yield decreased to 36% yield (3t). Notably, the catalytic system was also suitable for the diazoacetate. It has some effect on the yield when the ester group changed into isopropyl or tertbutyl group with larger steric hindrance (35%–64% yield, 3u-3y).
|
Download:
|
| Scheme 2. Substrate scope. Unless otherwise noted, the reactions were performed with 1 (0.1 mmol), 2 (0.4 mmol) and Al(OTf)3 (10 mol%) in DCE (0.8 mL) at 80 ℃ for 24 h under nitrogen. Yields of isolated product 3. | |
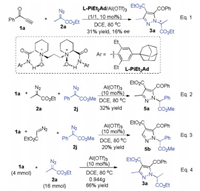
Based on this outcome, we tried to test the one-pot enantioselective N-H insertion to yield optically active product 3. Many kinds of chiral ligands, such as BINAP, Box, Pybox were used, the products were isolated in moderate yield but no enanatioselectivity (for details, see the Supporting information). Besides, a series of chiral N, N'-dioxide ligands that was developed in our group were screened, only the ligand L-PiEt2-1-Ad derived from (S)-pipecolic acid could get an enantioselectivity of 16% ee (Scheme 3, Eq. 1).
|
Download:
|
| Scheme 3. Other reactions. | |
In view that two molecules of α-diazoesters transferred into the product 3, we next subjected two different α-diazoesters into the catalyst system. As shown in Eq. 2, the reaction of alkynoe 1a, 2-diazopropanoate 2a, and 2-diazo-2-phenylacetate 2j delivered the desired pyrazole 5a, which is generate via [3 + 2] cycloaddition of 2a and N-H insertion into 2j. When ethyl 2-diazoacetate and diazophenylacetate 2j were mixed with ynone 1a, the pyrzaole product 5b was observed, which is the N-H insertion product with 2j (Eq. 3). These results were different from previous synthesis of pyrazoles using α-diazoarylacetate and methyl propiolate, in which diazophenylacetate participated in cycloaddition, following 1, 5-phenyl shift and without further N-H insertion reaction [10]. To show the synthetic utility of the cascade reactions, the gram scale experiment was performed. Alkynone 1a (4.0 mmol) reacted with ethyl 2-diazopropanoate 2a (16.0 mmol), providing the desired product 3a in 66% yield (0.944 g) (Eq. 4).
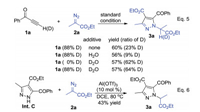
Next, deuterium-labelling experiments were carried out to probe into the rearrangement processes. The deuterium-labelled alkynone [D1]1a (88% D) or deuterated additives (D2O) were examined in the cascade reactions (Scheme 4, Eq. 5). When [D1]1a was used for the reaction in either the absence or presence of additive water, the product [D1]3a had low deuterium ratio at the chiral center. This data suggest that the proton does not originate from the alkynone. When 1a or [D1]1a was used with deuterated additives (D2O) in the reaction, [D1]3a is detected in dramatically increased deuterium ratio. It demonstrates that the proton can originate from external sources (trace amount of water containing in the catalytic system) for the two proton shift steps. Nevertheless, the extra water additive (exceeded 1.0 equiv.) decreased the isolated yield of the product 3a.
|
Download:
|
| Scheme 4. Control experiments. | |
Kinetic experiments were conducted with 1a and 2a through the operando IR instrument (for detail, see the Supporting information). According to the initial rates based on various concentrations of each component involved in the reaction, the reaction showed clear first-order dependence on the substrate 1a, 2a and the catalyst Al(OTf)3. Int. C was synthesized based previous reported [11], and was used to react with 2a at standard conditions to give the product 3a with 43% yield (Scheme 4, Eq. 6). No product detected without a catalyst. These feature of kinetics and control experiments indicate that the cycloaddition of diazo substrate, ynone in the assistance of Al(OTf)3 is likely the rate-limiting step.
Based on the above experiments, the mechanism of this reaction was proposed (Scheme 5). Firstly, Al(OTf)3 actives alkynone 1 by coordinating its benzoyl group to lower the LUMO of alkyne, then cycloaddition with alkyl α-diazoacetate affords the key 3-methyl-3H-pyrazole-3-carboxylate A. The ester group has higher migratory aptitude than methyl group or hydrogen, and then 1, 5-ester shift leads to the formation of minor trisubstituted pyrazole 4 or the major intermediate B. The structure of the product 4a was confirmed by single-crystal X-ray diffraction analysis. The supplementary crystallographic data of 4a (CCDC: 2008350) can be obtained free of charge from The Cambridge Crystallographic Data Centre. Water assisted intermolecular 1, 3-proton shift yields the N-H pyrazole Int C. The NH-based pyrazole C from methyl 2-diazobutanonate and ynone 1a was detected as the major product (see Scheme S1 in Supporting information for details). Finally, a Lewis acid assisted N-H insertion occurs readily to give the final product of N-alkyl 3-alkyl-4-ester-5-benzoyl substituted pyrazole 3.

|
Download:
|
| Scheme 5. Plausible mechanism. | |
In summary, we have developed an Al(OTf)3-promoted [3 + 2] cycloaddition/rearrangement/N-H insertion cascade reaction of alkynones and α-diazoesters. The reaction afforded a series of four-substituted pyrazole derivatives in moderate to good yield. Further studies on the utility of this method are in progress.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentThis work is supported by the National Natural Science Foundation of China (Nos. 21625205 and U19A2014).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.11.053.
| [1] |
(a) L.Pizzutia, A.G.Barschakb, S.Moura, etal., Curr.Org.Chem.18 (2014) 115-126;
(b) M.S. Moreno, C. Marín, J. Pitarch, et al., J. Med. Chem. 55 (2012) 4231-4243; (c) A. Ansari, A. Ali, M. Asif, Shamsuzzaman, New J. Chem. 41 (2017) 16-41; (d) M.E. Monteiro, G. Lechuga, M.C.S. Pereira, et al., Eur. J. Med. Chem.182 (2019) 111610-111623; (e) M.S.D. Santos, M.L.V. Oliveira, M.M.C. Cavalheiro, et al., Bioorg. Med. Chem. Lett. 21 (2011) 7451-7454; (f)B.F.Ruan, Y.Z.Zhu, W.D.Liu, B.A.Song, Y.P.Tian, Eur.J.Med.Chem.72 (2014) 46-51; (g)M.F.Khan, M.M.Alam, M.Shaquiquzzaman, etal., Eur.J.Med.Chem.120 (2016) 170-201; (h) J.V. Faria, P.F. Vegi, A.M.R. Bernardino, et al., Bioorg. Med. Chem. 25 (2017) 5891-5903. |
| [2] |
(a) L. Leroy, S. Cousin, A. Italiano, Eur. J. Cancer 81 (2017) 102-105; (b) J.A. Balfour, H.M. Bryson, R.N. Brogden, Drugs 51 (1996) 99-136; (c) M.M. Buckley, R.N. Brogden, K.L. Goa, et al., Drugs 44 (1992) 408-444; (d) A. Sadurn, G. Kehr, R. Gilmour, et al., Chem. Eur. J. 24 (2018) 2832-2836; (e) M.S. McClure, M.B. Berry, J. Powers, et al., Eur. J. Org. Chem. 19 (2012) 3561-3565; (f) D.W. Graham, W.T. Ashton, E.F. Rogers, et al., J. Med. Chem. 30 (1987) 1074-1090; (g) P.G. Middleton, M.A. Mall, R. Jain, et al., N. Engl. J. Med. 381 (2019) 1809-1819; (h) T. Nishina, S. Takahashi, T. Doi, et al., Invest. New Drugs 36 (2018) 424-434. |
| [3] |
(a) S. Fustero, M.S. Rosello, P. Barrio, A.S. Fuentes, Chem. Rev. 111 (2011) 6984-7034; (b) K. Karrouchi, M. Ansar, et al., Molecules 23 (2018) 134-220; (c) J.S.S. Neto, G. Zeni, Chem. Eur. J. 26 (2020) 8175-8189; (d) Y.L. Janin, Chem. Rev. 112 (2012) 3924-3958; (e) B. Eftekhari-Sis, M. Zirak, A. Akbari, Chem. Rev. 113 (2013) 2958-3043. |
| [4] |
(a) A. Lévai, A.M.S. Silva, J. Jeko, et al., Eur. J. Org. Chem. 12 (2006) 2825-2832; (b) S.K. Singh, M.S. Reddy, Y.K. Rao, et al., Tetrahedron Lett. 45 (2004) 7679-7682; (c) Z.X. Wang, H.L. Qin, Green Chem. 6 (2004) 90-92; (d) M.C. Bagley, M.C. Lubinu, C. Mason, Synlett 5 (2007) 704-708; (e) G. Cabarrocas, M. Ventura, J.M. Villalgordo, Tetrahedron: Asymmetry 11 (2000) 2483-2493; (f) D.B. Grotjahn, S. Van, L. Mejorad, et al., J. Org. Chem. 67 (2002) 9200-9209. |
| [5] |
(a) K. Mohanan, A.R. Martin, J.J. Vasseur, et al., Angew. Chem. Int. Ed. 49 (2010) 3196-3199; (b) Z. Chen, Y. Zhang, J. Nie, J.A. Ma, Org. Lett. 20 (2018) 2120-2124; (c) P.K. Mykhailiuk, Eur. J. Org. Chem. 33 (2015) 7235-7239. |
| [6] |
D. Drikermann, V. Kerndl, H. Görls, I. Vilotijevic, Synlett 31 (2020) 1158-1162. DOI:10.1055/s-0040-1707111 |
| [7] |
(a) M.P. Doyle, M.A. McKervey, T. Ye, Modern Catalytic Methods for Organic Synthesis With Diazo Compounds: From Cyclopropanes to Ylides, Wiley, New York, 1998; (b) A. Ford, H. Miel, M.A. McKervey, et al., Chem. Rev. 115 (2015) 9981-10080. |
| [8] |
(a) A. Padwa, W.H. Pearson, Synthetic Applications of 1, 3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, John Wiley and Sons, New York, 2002; (b) A. Arrieta, J.R. Carrillo, A. Morenn, et al., Tetrahedron 54 (1998) 13167-13168; (c) S. Kobayashi, R. Hirabayashi, Y. Yamashita, etal., Tetrahedron Lett. 44 (2003) 3351-3354; (d) L. Wu, M. Shi, J. Org. Chem. 75 (2010) 2296-2301; (e) N. Jiang, C.J. Li, Chem. Commun. (2004) 394-395; (f) Y. Li, L. Liu, J. Zhang, Org. Lett. 20 (2018) 6444-6448. |
| [9] |
(a) P.R. Krishna, E.R. Sekhar, F. Mongin, Tetrahedron Lett. 49 (2008) 6768-6772; (b) X. Qi, J.M. Ready, Angew. Chem. Int. Ed. 46 (2007) 3242-3244; (c) N.A. McGrath, R.T. Raines, Chem. Sci. 3 (2012) 3237-3240; (d) P. Zhao, S. Wu, C. Ke, X. Liu, X. Feng, Chem. Commun. 54 (2018) 9837-9840. |
| [10] |
X. Yi, J. Feng, F. Huang, J.B. Baell, Chem. Commun. 56 (2020) 1243-1246. DOI:10.1039/C9CC08389H |
| [11] |
J. Shao, W. Chen, M. Zhao, et al., Org. Lett. 20 (2018) 3992-3995. DOI:10.1021/acs.orglett.8b01562 |
 2021, Vol. 32
2021, Vol. 32