b State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics & Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, Xiamen 361102, China
The normal cornea maintains transparency and avascularity to serve as a refractive tissue for eyes [1]. Corneal neovascularization (CNV) occurs in various pathological conditions, and has become a sight-threatening condition that affects 4% of the US population [2]. In China, alkali burn, long-term wear of contact lenses, herpes simplex virus infection, limbal stem cell deficiency and graft rejection are the most common causes in clinical cases [3].
Current treatments for CNV include medical treatment and surgical treatment. In medical treatment, topical administration of steroids and nonsteroidal anti-inflammatory agents (NSAIDs) remains top priority, while long-term use of them may result in glaucoma, cataracts and super infection [4]. Anti-VEGF agents like VEGF neutralizing antibodies (Bevacizumab) [5] and VEGF silencing RNA (siRNA) [6] are also applied. However, anti-VEGF therapy can hardly destroy long-standing vessels and frequent injections may be required [7]. As for surgical treatment, laser thermal cauterization by argon laser, yellow laser and Nd: YAG laser have been applied through light-induced thermal damages [8]. This strategy can achieve immediate CNV occlusion, however, some complications like corneal hemorrhage, corneal thinning and iris atrophy are often reported [9, 10]. Therefore, CNV treatments based on laser irridiation are worthy of more improvements to achieve better therapeutic effect. As for CNV treatment in China, some traditional Chinese medical therapies like acupuncture treatment and drug agents of Glycyrrhiza glabra and catalpol have also been reported to eliminate CNV to some degree [11, 12].
Nowadays, phototherapy (PT) including photothermal therapy (PTT) and photodynamic therapy (PDT) is emerging as a modern medical technique to treat various diseases [13]. PT is widely known for its diverse charecteristics like non-invasiveness, precise selectivity, deep tissue penetration, and minimal side effects. Meanwhile, PT has also been used to treat CNV with some promising achivements: (ⅰ) expeditious treatment in case of deterioration; (ⅱ) accurate and safe CNV elimination; (ⅲ) feasibility in clinical translation. In PDT for CNV treatment, Maurizio et al. reported a verteporfin-based PDT in 2 patients with CNV [14]. The results showed that successful photothrombosis of CNV was obtained immediately after treatment and complete regression of new vessels was observed after one repeated session in both cases. In another clinical study, You et al. constructed a verteporfin-based PDT with subconjunctival injection of bevacizumab in 12 patients with CNV [15]. They reported decrease of CNV in all cases and complete vascular occlusion in 66.7% after a single session in 1 month, then 91.9% achieved CNV regression after treatment for 1.5 years. In PTT for CNV treatment, one case has reported the short-term regression of CNV with combination therapy of argon green laser photocoagulation and subconjunctival bevacizumab of a 49 year-old male [16]. One case has reported that deep CNV of a 24 year-old male was successfully managed using a combination of subconjunctival bevacizumab and argon laser photocoagulation [17]. In another study, frequency-doubled Nd: YAG (532 nm) laser photocoagulation showed an effective reduction in the area of CNV from 31.94% to 17.63% at the end of 3 months [18]. These findings indicated that PT could be a promising strategy in CNV treatment. However, verteporfin has unknown system damage from intravenous infusion [19]. Other PDT drug such as dihematoporphyrin ether (DHE) also demonstrates efficacy but has more complications like photoxic reactions and transient angioedema [20]. Therefore, suitable PT agents in CNV treatment remain to be developed and topical administration like eyedrops or subconjunctival injection maybe more appropriate than systemic administration.
Over the past years, nanotechnology has developed extensively and gained great success. [21, 22]. Also, nanoparticle-mediated drug delivery are applied to CNV elimination due to its multifunctional properties: (ⅰ) targeted delivery with less accumulation in normal tissues to reduce toxic damages; (ⅱ) enhanced drug permeation and retention time, and controlled drug release; (ⅲ) therapeutic effects combined with monitoring and diagnostic functions. Pradhan et al. reported that eyedrops of curcumin-loaded MePEG-PCL nanoparticles exhibited enhanced retention of curcumin in corneas and improved the prevention of CNV over free curcumin [23]. In another study, Usui et al. encapsulated dendrimer porphyrin (DP, free DP) into polymeric micelles (DP-micelle) [24]. They found that fluorescence of DP-micelle was stronger than that of DP in neovascularized area. After PDT treatment, mean residual rate of CNV in DP-micelle-treated mice was considerably lower compared with DP-treated mice. Therefore, nanoparticle-mediated drug delivery combined with PDT maybe potential candidate for prevention of CNV.
In view of the fact that both PDT and thermal therapy could eliminate CNV through distinctive mechanisms, the PDT/PTT-combined effects can be achieved simultaneously with one irradiation to improve efficiency. Indocyanine green (ICG) is a near-infrared (NIR) dye approved by FDA for clinical use [25]. ICG is widely used as PT agents because of its ability to transform NIR laser into heat and meanwhile generate ROS to accomplish PDT and PTT together. However, several defects like unstability, non-selectivity and low permeability have limited its application [26]. Recently, the interaction between ICG and metal ion was designed into a nanocomposite to achieve better PT efficacy. Chu et al. found that DPA-Zn could interact with the sulfonate anions of ICG to prepare for a DPA-Zn/ICG metal-organic nanoparticle (named nanoICG) [27]. Compared with free ICG, nanoICG exhibited advantages of high stability, precise selectivity, strong permeability and enhanced PT efficacy to achieve better therapeutic effect. Simultaneously, nanoICG was modified with RGD peptide by the addition of bis(DPA-Zn)-RGD (R-nanoICG), which could selectively target corneal vessels without residua in normal tissues, due to the angiogenesis-targeting effect of RGD [28].
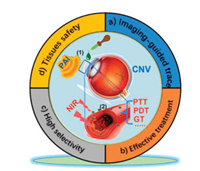
In addition, accurate and timely imaging is necessary in diagnosis and drug tracing [29]. Photoacoustic imaging (PAI) is a hybrid and non-invasive imaging modality to monitor structural and functional changes in tissues by converting absorbed laser into ultrasound signal [30]. PAI exhibits multiple advantages, such as safe, high sensitivity and resolution, real-time imaging. In CNV model, hemoglobin present in blood vessels can function as endogenous chromophores due to their strong light absorption in certain optical window without using exogenous agents. Hence, PAI is suitable for ophthalmic imaging to trace drugs and exhibit eyeballs in detail via the combination with photoacoustic drugs. Moreover, PAI can diagnose oxygen saturation (o.s.) of hemoglobin, which is observed exclusively in neovascularized corneas, and thus PA-o.s. imaging can locate blood vessels from CNV [27]. Since R-nanoICG possessed PA property to serve as a PAI agent, after topical administration of R-nanoICG via eyedrops or subconjunctival injection, PAI could trace its accumulation process in CNV. When neovascularized areas were enriched with the highest degree of R-nanoICG, an 808 nm laser was irradiated to excite a series of reactions from the nasal side to the temporal side of the cornea, and then repeated in reverse to avoid retina injuries. R-nanoICG could be loaded with siRNA to silence target genes of survivin and heat shock protein 70 (HSP70), and thus accomplish gene therapy (GT). PT combined with GT showed a synergistic inhibitory effect in CNV treatment. Taken together, the novel R-nanoICG/siRNA developed in this study could be an ideal nanotheranostics for real-time in vivo multimodal PAI and photo/gene combinatorial strategy in CNV treatment (Fig. 1).
|
Download:
|
| Fig. 1. A simple but powerful theranostic strategy is proposed and validated for PT/GT-combined treatment in CNV inhibition, featuring noninvasive PAI-guided trace, effective treatment, high selectivity and tissue safety. | |
Among these traditional CNV treatments, anti-inflammatory and anti-VEGF medications remain the first choice. Recently, several novel strategies have also gained success. For example, gene-based antiangiogenic therapy like nanoparticle-mediated delivery of short hairpin RNA (shRNA) against VEGF-A could regress CNV [31]. In Chu et al.'s study, the synthesis of R-nanoICG is a promising delivery system through the interaction between organic ions and multiple PT agents with various advantages like efficiency, selectivity, non-toxicity in normal tissues and imaging-guided functions. It is believed that this photoacoustic-based strategy can provide more opportunities for further research in CNV treatment. In the future, with the development of technology and continuous research on the mechanism of CNV in-depth, we believe that more efficient, safe and potent anti-CNV strategies can be applied from bench to bedside.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis study was supported in part by the National Key R & D Program of China (Nos. 2018YFA0107301 and 2018YFA0107304), the National Natural Science Foundation of China (NSFC, Nos. 81770894, 81901876, 81470602 and 81330022) and the Fundamental Research Funds for the Central Universities (Nos. 20720190088 and 20720200019), the Medical and Health Key Project of Xiamen (No. 3502Z20191106), and the Program for New Century Excellent Talents in University, China (No. NCET-13-0502).
| [1] |
Z. Sharif, W. Sharif, Rom. J. Ophthalmol. 63 (2019) 15-22. DOI:10.22336/rjo.2019.4 |
| [2] |
J.H. Chang, E.E. Gabison, T. Kato, et al., Surv. Ophthalmol. 57 (2012) 415-429. DOI:10.1016/j.survophthal.2012.01.007 |
| [3] |
M. Chen, L. Bao, M. Zhao, et al., Front. Pharmacol. 11 (2020) 111-116. DOI:10.3389/fphar.2020.00111 |
| [4] |
X. Liu, S. Wang, X. Wang, et al., Chem. Biol. Drug Des. 90 (2017) 653-664. DOI:10.1111/cbdd.13018 |
| [5] |
J.H. Park, C.K. Joo, S.K. Chung, Cornea 34 (2015) 449-455. DOI:10.1097/ICO.0000000000000336 |
| [6] |
M. Murata, T. Takanami, S. Shimizu, et al., Curr. Eye Res. 31 (2006) 171-180. DOI:10.1080/02713680500514636 |
| [7] |
O.B. Voiculescu, L.M. Voinea, C. Alexandrescu, J. Med. Life 8 (2015) 444-448. |
| [8] |
D. Gupta, C. Illingworth, Cornea 30 (2011) 927-938. DOI:10.1097/ICO.0b013e318201405a |
| [9] |
D. Roshandel, M. Eslani, A. Baradaran-Rafii, et al., Ocul. Surf. 16 (2018) 398-414. DOI:10.1016/j.jtos.2018.06.004 |
| [10] |
Y. Benayoun, F. Petellat, O. Leclerc, et al., J. Fr. Ophtalmol. 38 (2015) 996-1008. DOI:10.1016/j.jfo.2015.09.006 |
| [11] |
Y. Han, M. Shen, L.Y. Tang, et al., Mol. Med. Rep. 17 (2017) 2187-2194. |
| [12] |
S.L. Shah, F. Wahid, N. Khan, et al., Evid. Based Complement. Alternat. Med. 2018 (2018) 101-108. |
| [13] |
E.A. Gordon Spratt, L.V. Gorcey, N.A. Soter, et al., Br. J. Dermatol. 173 (2015) 19-30. DOI:10.1111/bjd.13544 |
| [14] |
M. Fossarello, E. Peiretti, I. Zucca, et al., Cornea 22 (2003) 485-488. DOI:10.1097/00003226-200307000-00018 |
| [15] |
I.C. You, S.K. Im, S.H. Lee, et al., Cornea 30 (2011) 30-33. DOI:10.1097/ICO.0b013e3181dc81a0 |
| [16] |
N. Anand, J.J. Reidy, K.M. Riaz, Int. Med. Case Rep. J. 12 (2019) 89-92. DOI:10.2147/IMCRJ.S195990 |
| [17] |
M. Lakshmipathy, P. Susvar, K. Popet, IndianJ.Ophthalmol. 67 (2019) 1193-1194. DOI:10.4103/ijo.IJO_1583_18 |
| [18] |
J. Kumar, A. Gehra, N. Sirohi, J. Clin. Diagn. Res. 10 (2016) 1-4. DOI:10.1111/crj.12367 |
| [19] |
F. Ziemssen, H. Heimann, F.A. Lattanzio, et al., Expert Opin. Drug Metab. Toxicol. 8 (2012) 1023-1041. DOI:10.1517/17425255.2012.701617 |
| [20] |
J.D. Sheppard, R.J. Epstein, F.A. Lattanzio, et al., Am. J. Ophthalmol. 141 (2006) 524-529. DOI:10.1016/j.ajo.2005.11.003 |
| [21] |
H. Chen, H. Cheng, W. Wu, et al., Chin. Chem. Lett. 31 (2020) 1375-1381. DOI:10.1016/j.cclet.2020.03.024 |
| [22] |
D.R. Janagam, L. Wu, T.L. Lowe, Adv. Drug Deliv. Rev. 122 (2017) 31-64. DOI:10.1016/j.addr.2017.04.001 |
| [23] |
N. Pradhan, R. Guha, S. Chowdhury, et al., J. Mol. Med. (Berl.) 93 (2015) 1095-1106. DOI:10.1007/s00109-015-1277-z |
| [24] |
T. Usui, K. Sugisaki, S. Amano, et al., Cornea 24 (2005) 39-42. DOI:10.1097/01.ico.0000138836.45070.0f |
| [25] |
C. Egloff-Juras, L. Bezdetnaya, G. Dolivet, et al., Int. J. Nanomed. 14 (2019) 7823-7838. DOI:10.2147/IJN.S207486 |
| [26] |
M.B. Reinhart, C.R. Huntington, L.J. Blair, et al., Surg. Innov. 23 (2016) 166-175. DOI:10.1177/1553350615604053 |
| [27] |
C. Chu, J. Yu, E. Ren, et al., Adv. Sci. 7 (2020) 2000346. DOI:10.1002/advs.202000346 |
| [28] |
S. Katsamakas, T. Chatzisideri, S. Thysiadis, et al., Future Med. Chem. 9 (2017) 579-604. DOI:10.4155/fmc-2017-0008 |
| [29] |
E. Ren, J. Wang, G. Liu, Chin. Chem. Lett. 28 (2017) 1799-1800. DOI:10.1016/j.cclet.2017.07.015 |
| [30] |
P. Yang, Y. Men, Y. Tian, et al., ACS Appl. Mater. Interfaces 11 (2019) 11209-11219. DOI:10.1021/acsami.9b01286 |
| [31] |
Y. Qazi, B. Stagg, N. Singh, et al., Invest. Ophthalmol. Vis. Sci. 53 (2012) 2837-2844. DOI:10.1167/iovs.11-9139 |
 2021, Vol. 32
2021, Vol. 32 

