b Graduate School, University of Chinese Academy of Sciences, Beijing 100049, China;
c Beijing Key Laboratory of Energy Conversion and Storage Materials, College of Chemistry, Beijing Normal University, Beijing 100875, China;
d State Key Laboratory of Toxicology and Medical Countermeasures, and Laboratory of Toxicant Analysis, Academy of Military Medical Sciences, Beijing 100850, China
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI MS) is an effective tool for analysis of nonvolatile compounds, such as proteins [1], peptides [2], carbohydrates [3], and synthetic polymers [4-7]. MALDI MS has its extraordinary advantages including high throughput, no cross contamination, low fragmentation and high sensitivity [8-11]. In MALDI MS analysis, analyte is usually mixed with matrix, and then dropped on the sample target. After the mixture is dried, the sample target is loaded into the high vacuum ion source of the instrument for MALDI MS analysis. In general, it only takes several minutes to analyze the sample, which is very convenient. However, for analysis of volatile compounds, the analyte can evaporate easily during the drying process and in the high vacuum circumstance of the ion source, which leads to the absence of analyte on the sample target, consequently resulting in a failure of the characterization of the volatile compounds by MALDI MS. Traditionally, volatile compounds are enriched by solid phase microextraction device and then analyzed by gas chromatography-electron ionization-mass spectrometry (GC-EI-MS), which is a time-consuming procedure [12, 13]. As a result, the approach of MALDI MS in analysis of volatile compounds is worth exploring.
Matrix is vital for the characterization of samples by MALDI MS [14]. Therefore, for a long time the development of new matrix has been a research hotspot in MALDI MS. Some organic compounds, like α-cyano-4-hydroxycinnamic acid (CHCA) [15], sinapic acid (SA) [16], 2, 5-dihydroxybenzoic acid (DHB) [17], and various materials with different composition as new matrices, such as porous silicon [18-20], metal nanoparticles [21, 22], metal oxides [23, 24] and carbon-based materials [25-29], have been developed, which is highly successful to expend the applications of MALDI MS [5, 30-33]. Nevertheless, matrix used to analyze volatile compounds has not been reported so far.
In this work, we designed a coordination compound derived Co and N doped porous carbon (Co-NC) material, which has relatively large specific surface area, strong interaction force with volatile compounds, and stable physical and chemical property [34]. This material can strongly adsorb and enrich the volatile compounds in air, and can hold them firmly even in the high vacuum circumstance. More importantly, this material can be used as matrix of MALDI MS for desorption and ionization of volatile compounds. This Co-NC material as new matrix provides the ability of MALDI MS to analyze volatile compounds, and tremendously extends the application of MALDI MS.
The aldimine condensation usually have high reaction rate, and the product of the reaction, Schiff base, is very stable [35]. In this work, 2-aminobenzimidazole and 2-pyridinecarboxaldehyde were mixed and quickly reacted under the catalysis of CoCl2·6H2O. Color transformation of the solution from dark blue to black during the reaction indicated the generation of new compound, and elemental analysis (C 48.97, H 3.97, N 17.25) illuminated the high concentration of N in the compound.
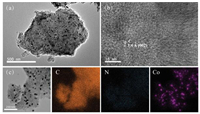
After calcination, the morphology and elemental composition were characterized. Transmission electron microscopy (TEM) image (Fig. 1a) exhibited that Co-NC was the folded and lamellar mixed structure with many pores. High resolution (HR)-TEM (HR-TEM) image of Co-NC (Fig. 1b) showed the abundant graphitic carbon structure with interplane spacing of (002) crystal lattice (3.4 Å), which could be attributed to the catalytic graphitization behavior of Co nanoparticles (NPs) in the structure [36-38]. Elemental mapping (Fig. 1c) showed that Co-NC was mainly composed of C, N, and Co, among which N and Co were dispersed uniformly throughout the carbon matrix.
|
Download:
|
| Fig. 1. (a) TEM image, (b) HR-TEM image, and (c) elemental mapping of C, N, and Co of the Co-NC material, showing graphitic carbon structures and homogeneous distribution of Co and N in the carbon layers. | |
The chemical state of C, N and Co in Co-NC material was analyzed by X-ray photoelectron spectroscopy (XPS). The XPS demonstrated that the binding energy for carbon element was 284.22 eV (Figs. S1a and b in Supporting information), suggesting most carbon atoms use sp2 hybrid orbit to bind with other atoms in the structure. The result demonstrated that Co-NC was a highly graphitized material, which was consistent with the TEM data. The XPS spectra for N 1s in Co-NC present four types of nitrogen species (Figs. S1a and c in Supporting information): dominant graphitic-N (401.1 eV) and Co-N (399.2 eV), pyridinic-N (398.4 eV) as well as pyrrolic-N (400.2 eV) [35, 38]. These kinds of nitrogen atoms could be used as Lewis base, which had the ability to combine protons when interacted with analytes. Co was also well doped in the graphic layers and had interaction with C and N atoms in the structure (Figs. S1a and d in Supporting information). The role of Co, we suspected, might increase the density and stability of the Co-NC material so that Co-NC did not contaminate the ion source.
N2 adsorption–desorption experiments were performed to obtain the Brunauer-Emmett-Teller (BET) specific surface area of synthesized Co-NC material, which was corresponding to 355 m2/g (Fig. S2a in Supporting information) with many mesoporous (38 Å). To be used as a matrix in MALDI analysis, the light absorption property is also important. As shown in the UV–vis-near IR absorption spectrum (Fig. S2b in Supporting information), this material presented broad absorption from 240 nm to 700 nm. As a result, when exposed to 355 nm laser in the ion source of MS, Co-NC could absorb the energy of the laser, and then transfer the energy to the analyte for desorption and ionization of analyte.
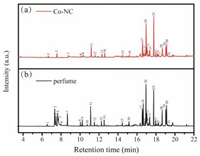
The adsorption capacity of Co-NC was investigated. The Co-NC material (5 mg) was incubated with the perfume (DKNY) (10 μL) in a 160 ℃ airtight environment (40 mL) for 30 min. The volatile compounds in perfume adsorbed in the Co-NC were eluted by 100 μL of ethanol, and then 0.2 μL were loaded into a GC-EI mass spectrometer for analysis. The results were shown in Fig. 2a and Table S1 (Supporting information). Totally, 26 compounds, including D-limonene, linalool, etc., were absorbed by Co-NC and detected by GC-EI-MS. Direct analysis of the perfume (10 μL perfume diluted in 100 μL of ethanol, then 0.2 μL was injected to make the analysis) by GC-EI-MS showed 31 compounds were detected (Fig. 2b). Although 5 compounds, mainly aliphatic alcohols, could not be adsorbed by Co-NC under this condition, the Co-NC showed the strong adsorption capacity for many kinds of volatile compounds such as alkene, higher alcohols and esters. The failure of adsorption for those aliphatic alcohols might attributed to their high polarity. The adsorption performance of Co-NC was further compared with the classical adsorbent, activated carbon. The results demonstrated that Co-NC had similar adsorption capacity to that of the activated carbon (Fig. S3 in Supporting information). The strong adsorption capacity of Co-NC to volatile compounds may be originated from the graphitized carbon and porous structure in the material.
|
Download:
|
| Fig. 2. GC-EI-MS spectra of volatile compounds in perfume with (a) or without (b) the Co-NC material as absorbent. | |
The performance of Co-NC as matrix of MALDI MS was investigated. Cytidine and taurine, as examples, were analyzed by using Co-NC as matrix of MALDI MS. Traditional organic matrices, CHCA and 9-acridinamine (9-AA), were also chosen for comparison, and the results were shown in Fig. S4 (Supporting information). Firstly, for these 2 compounds, no mass spectra signals can be obtained without matrix (Figs. S4a and d). By using Co-NC as matrix, the sodium and potassium adduct signals of cytidine with m/z at 266.1 and 282.0 respectively were obtained in positive ionization mode without background ions interference (Fig. S4b). Likewise, deprotonated taurine signal with m/z at 124.0 was clearly obtained in negative ionization mode (Fig. S4e). On the contrary, when using CHCA and 9-AA as matrix in the positive and negative mode respectively, strong background interferences were generated (Figs. S4c and f), and blurred the signal of the analyte. Taken together, Co-NC is a successful matrix of MALDI MS, which is available for both positive and negative ionization modes without background interferences in the low-mass region (< 500 Da).
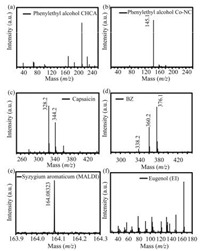
Further, the performance of Co-NC, simultaneously acting as adsorbent of volatile compounds and matrix of MALDI MS, was investigated. Two volatile compounds, benzyl alcohol (bp: 205.7 ℃, vp: 0.13 kpa (58 ℃)) and phenethyl alcohol (bp: 219.5 ℃, vp: 0.13 kpa (58 ℃)), with low boiling point (bp) and relative high vapor pressure (vp), were chosen as examples. Obviously, no signal of volatile compounds could be observed when using CHCA as matrix (Fig. 3a). On the contrary, the signal of benzyl alcohol (Fig. S5 in Supporting information) and phenethyl alcohol (Fig. 3b) is easily captured using Co-NC as matrix, indicating Co-NC had a strong interaction with analyte even in the high vacuum environment, and could be used as adsorbent and matrix for MALDI MS analysis of volatile compounds. A comparison with classical absorbent (activated carbon) was also performed. However, no signal was obtained. This may be attributed to the low ionization efficiency of analyte using activated carbon as matrix.
|
Download:
|
| Fig. 3. Mass spectra of volatile compounds. (a) Phenylethyl alcohol, CHCA used as matrix. (b) Phenylethyl alcohol, Co-NC used as matrix. (c) Capsaicin, Co-NC used as matrix. (d) 3-Quinuclidinyl benzilate (BZ), Co-NC used as matrix. (e) The concentration from Syzygium aromaticum, Co-NC used as matrix. (a–e) were obtained by MALDI MS. (f) The concentrates from Syzygium aromaticum, obtained by GC-EI-MS. | |
One riot-control agent, capsaicin, and one incapacitating agent, 3-quinuclidinyl benzilate (BZ), were also used as examples to test the capability of Co-NC as adsorbent and matrix. The vapourable capsaicin and BZ were adsorbed and analyzed. The results were shown in Figs. 3c and d, in which the sodium adduct and potassium adduct signals for both of the compounds were presented in the spectra. This phenomenon showed that Co-NC can absorb capsaicin and BZ, even though the two incapacitating agents presented solid state under normal condition. In addition, the Co-NC can be used as matrix for MALDI MS analysis of capsaicin and BZ.
Volatile compounds from herbs have long-time been regarded as fingerprint of their parent botanies, which could be also used as a criterion for evaluating the quality of the herb. As an application example, the Co-NC was used as adsorbent and matrix to analyze them. In a typical process, Co-NC was dropped on the stainless-steel target plate followed by incubation with 1 g Syzygium aromaticum herb for 3 h, and then MALDI-FTICR-MS was performed to identify the elemental composition of the components captured by Co-NC. As demonstrated in the spectrum (Fig. 3e), peak with m/z at 164.08323 (C10H12O2, M+) was observed, which is in accorded with the main volatile oil component eugenol. To further confirm the component captured by Co-NC, 5 mg Co-NC was eluted by 100 μL of ethanol, and the solution was analyzed by GC-EI-MS in succession. The EI mass spectrum (Fig. 3f) for the main component showed 95% similarity with the standard spectrum of eugenol, indicating that Co-NC had the capacity to adsorb the main volatile components from syzygium aromaticum, and more importantly, could be further used as matrix to identify the compounds using MALDI-MS.
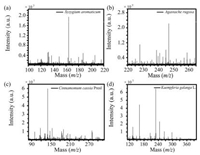
Using Co-NC as adsorbent and matrix, the volatile compounds in Syzygium aromaticum, Agastache rugosa, Cinnamomum cassia Presl, and Kaempferia galanga L. were analyzed by MALDI MS. The main components in these four herbs were identified using accurate molecular mass obtained by MALDI-FTICR-MS (Table S2 in Suppporting information) (Fig. 4). The principal component analysis (PCA) showed that the volatile compounds from different herbs were significantly different, and can be used for distinguishing herbs. As expected, it shows completed and clear classification of all the four herbs, from the PCA result in Fig. S6 (Supporting information).
|
Download:
|
| Fig. 4. MALDI FTICR MS spectra of four herbs. (a) Syzygium aromaticum, (b) Agastache rugosa, (c) Cinnamomum cassia Presl, and (d) Kaempferia galanga L. | |
The adsorption rate of Co-NC for volatile compounds was then investigated. Coumarin ([M+H+]: 147.04406, [M + Na+]: 169.02603), a substance with strong odor and easy sublimation, was selected. Co-NC was firstly dropped onto to the stainless-steel target plate, and then 100 mg solid coumarin and the target were placed into a sealed beaker for 1-12 h respectively. And the target was loaded into the ion source to be analyzed. The results (Fig. S7 in Supporting information) revealed that the amount of adsorption was linearly increased in the first three hours, and then saturated basically in 5 h. The findings may also be a reference for the quantitative analysis of volatile compounds in the environment.
In summary, focusing on the analysis of volatile compounds by MALDI MS, we designed a new Co and N doped porous (38 Å) carbon matrix, Co-NC. The experimental results demonstrate that the Co-NC has relatively large specific surface area (355 m2/g) and strong interaction force with volatile compounds, which can strongly adsorb and enrich the volatile compounds in perfume and herbs, and can hold them even in the high vacuum circumstance. The Co-NC has ultraviolet (355 nm) absorption, and can be used as matrix of MALDI MS for desorption and ionization of lots of volatile compounds, like higher alcohols and esters, aromatic alcohol, etc. Co-NC is available for both positive and negative ion detection modes without background interferences in the low-mass region (< 500 Da), overcoming the challenges of MALDI MS analysis of small molecule compounds. In a word, this Co-NC material as new matrix provides the ability of MALDI MS to analyze volatile compounds in a very convenient and time-saving way, tremendously extends the application of MALDI MS.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was supported by the National Key R & D Program of China (No. 2018YFA0506900) and National Natural Science Foundation of China (Nos. 21635008 and 21621062), and the Military Medicine and Healthy Major Project, China (No. AWS16J016).
Appendix A. Supplementary dataSupplementarymaterial related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.09.026.
| [1] |
R. Aebersold, M. Mann, Nature 422 (2003) 198-207. DOI:10.1038/nature01511 |
| [2] |
R.J. Goodwin, S.R. Pennington, A.R. Pitt, Proteomics 8 (2008) 3785-3800. DOI:10.1002/pmic.200800320 |
| [3] |
R. Chen, W. Xu, C. Xiong, X. Zhou, et al., Anal. Chem. 84 (2012) 465-469. DOI:10.1021/ac202438a |
| [4] |
M. Karas, F. Hillenkamp, Anal. Chem. 60 (1988) 2299-2301. DOI:10.1021/ac00171a028 |
| [5] |
R.C. Beavis, T. Chaudhary, B.T. Chait, Org. Mass Spectrom. 27 (1992) 156-158. DOI:10.1002/oms.1210270217 |
| [6] |
Y. Suzuki, M. Suzuki, Y. Nakahara, et al., Anal. Chem. 78 (2006) 2239-2243. DOI:10.1021/ac051841x |
| [7] |
J.J. Hu, F. Liu, H.X. Ju, Angew. Chem. Int. Ed. 55 (2016) 6667-6670. DOI:10.1002/anie.201601096 |
| [8] |
M. Kompauer, S. Heiles, B. Spengler, Nat. Methods 14 (2017) 1156-1158. DOI:10.1038/nmeth.4433 |
| [9] |
T. Wu, X.H. Yang, C.J. Zhang, Z.P. Wang, Y.P. Du, Chin. Chem. Lett. 27 (2016) 901-904. DOI:10.1016/j.cclet.2016.02.020 |
| [10] |
Z. Wu, N. Xu, W. Li, J.M. Lin, Chin. Chem. Lett. 30 (2019) 95-98. DOI:10.1016/j.cclet.2018.01.048 |
| [11] |
M. Kompauer, S. Heiles, B. Spengler, Nat. Methods 14 (2017) 90-96. DOI:10.1038/nmeth.4071 |
| [12] |
V. Kraujalyte, E. Leitner, P.R. Venskutonis, J. Agric. Food Chem. 61 (2013) 4728-4736. DOI:10.1021/jf400152x |
| [13] |
B. Siegmund, K. Urdl, A. Jurek, E. Leitner, J. Agric. Food Chem. 66 (2018) 2432-2442. DOI:10.1021/acs.jafc.6b05009 |
| [14] |
S. Mouradian, C.M. Nelson, L.M. Smith, J. Am. Chem. Soc. 118 (1996) 8639-8645. DOI:10.1021/ja953585j |
| [15] |
S.A. Schwartz, M.L. Reyzer, R.M. Caprioli, J. Mass Spectrom. 38 (2003) 699-708. DOI:10.1002/jms.505 |
| [16] |
R. Kruse, J.V. Sweedler, J. Am. Soc. Mass Spectrom. 14 (2003) 752-759. DOI:10.1016/S1044-0305(03)00288-5 |
| [17] |
Y.B. Wei, Y.Y. Zhang, Y. Lin, et al., Analyst 140 (2015) 1298-1305. DOI:10.1039/C4AN01964D |
| [18] |
S. Okuno, R. Arakawa, K. Okamoto, Y. Matsui, et al., Anal. Chem. 77 (2005) 5364-5369. DOI:10.1021/ac050504l |
| [19] |
S.A. Stopka, C. Rong, A.R. Korte, S. Yadavilli, et al., Angew. Chem. Int. Ed. 55 (2016) 4482-4486. DOI:10.1002/anie.201511691 |
| [20] |
T.R. Northen, O. Yanes, M.T. Northen, et al., Nature 449 (2007) 1033-1036. DOI:10.1038/nature06195 |
| [21] |
J.A. McLean, K.A. Stumpo, D.H. Russell, J. Am. Chem. Soc. 127 (2005) 5304-5305. DOI:10.1021/ja043907w |
| [22] |
H. Kawasaki, K. Nakai, R. Arakawa, et al., Anal. Chem. 84 (2012) 9268-9275. DOI:10.1021/ac302004g |
| [23] |
X.Q. Xu, C.H. Deng, M.X. Gao, et al., Adv. Mater. 18 (2006) 3289-3293. DOI:10.1002/adma.200601546 |
| [24] |
X.J. Yang, X.K. Hu, A.V. Loboda, et al., Adv. Mater. 22 (2010) 4520-4523. DOI:10.1002/adma.201001627 |
| [25] |
Y.K. Kim, H.K. Na, S.J. Kwack, et al., ACS Nano 5 (2011) 4550-4561. DOI:10.1021/nn200245v |
| [26] |
Q.H. Min, X.X. Zhang, X.Q. Chen, et al., Anal. Chem. 86 (2014) 9122-9130. DOI:10.1021/ac501943n |
| [27] |
Z. Lin, J.N. Zheng, G. Lin, et al., Anal. Chem. 87 (2015) 8005-8012. DOI:10.1021/acs.analchem.5b02066 |
| [28] |
Z.Y. Rao, F.L. Geng, Y.Q. Zhou, et al., Anal. Methods 9 (2017) 2014-2020. DOI:10.1039/C7AY00112F |
| [29] |
Z. Lin, J. Wu, Y.Q. Dong, et al., Anal. Chem. 90 (2018) 10872-10880. DOI:10.1021/acs.analchem.8b02362 |
| [30] |
M. Karas, H. Ehring, E. Nordhoff, et al., Org. Mass Spectrom. 28 (1993) 1476-1481. DOI:10.1002/oms.1210281219 |
| [31] |
J. Yao, J.R. Scott, M.K. Young, C.L. Wilkins, J. Am. Soc. Mass Spectrom. 9 (1998) 805-813. DOI:10.1016/S1044-0305(98)00046-4 |
| [32] |
B. You, N. Jiang, M. Sheng, et al., Chem. Mater. 27 (2015) 7636-7642. DOI:10.1021/acs.chemmater.5b02877 |
| [33] |
Y.H. Zhang, Y.Y. Song, J. Wu, et al., Chem Comm. 55 (2019) 3745-3748. DOI:10.1039/C9CC00384C |
| [34] |
D. Wang, S.M. Li, J.Q. Zheng, et al., Inorg. Chem. 56 (2017) 984-990. DOI:10.1021/acs.inorgchem.6b02784 |
| [35] |
Y.Z. Chen, C. Wang, Z.Y. Wu, et al., Adv. Mater. 27 (2015) 5010-5016. DOI:10.1002/adma.201502315 |
| [36] |
J. Tang, R.R. Salunkhe, J. Liu, et al., J. Am. Chem. Soc. 137 (2015) 1572-1580. DOI:10.1021/ja511539a |
| [37] |
J.S. Meng, C.J. Niu, L.H. Xu, et al., J. Am. Chem. Soc. 139 (2017) 8212-8221. DOI:10.1021/jacs.7b01942 |
| [38] |
S. Yang, L. Peng, P. Huang, et al., Angew. Chem. Int. Ed. 55 (2016) 4016-4020. DOI:10.1002/anie.201600455 |
 2021, Vol. 32
2021, Vol. 32 

