Stimuli-responsive supramolecular systems nowadays have gained intense research interests as they are potential building blocks for fabrication of memory storage and drug delivery systems, chemical sensors and molecular machines [1-4]. In this aspect, the mechanically interlock molecules (MIMs) are attractive entities because of their potential stimuli caused the shuttle behaviors, which enable their applications as molecular device and machine [5]. A plethora of external stimuli including temperature, pH, light and redox were commonly employed in the fabrication of such functional systems [6-12]. Among them, light is the most facile and promising external stimuli and has attracted considerable attenations [13-15]. Azobenzene was recognized as one of the key light responsive motifs among the photo active moieties because of light triggered trans/cis isomerization, which could render substantial structure and property change of azobenzene [15]. Therefore, azobenzene derivates have been widely employed as guest of light-responsive MIMs [16].
Traditional host molecules for fabricating rotaxanes included crown ethers, cyclodextrins, and cucurbit[n]urils [17]. The synthesis and photo-responsive property of cucurbit[7]uril and azobenzene based [3]rotaxane has been reported. The systems exhibited molecular shuttle property upon irradiation of UV/vis light [18]. The novel macrocyclic structure of pillar[n]arenes (n = 5, 6), first reported in 2008 and described as "fascinating cyclophanes with a bright future", have been proved to be the promising candidate host for rotaxane and catananes [15]. Recently, some rotaxanes composed of azobenzene derivates and pillar[n]arenes hav been reported in literatures [19-21]. The photo-switch properties together with the function exploration of pillar[n]arenes-based azobenzene-bearing rotaxanes received considerable attentions [15]. Yet, current related investigations mainly focused on pillar[n]arene (n = 5, 6) based azobenzene bridged [1] and [2]rotaxanes. The light-responsive property of bis-pillar[n]arene and azobenzene based [3]rotaxanes remain much unexplored. Herein, we report the design, syntheses and photo-switch property of pillar[5]arene-based azobenzene-bridged [3]rotaxanes.
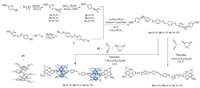
We used a straight-forward strategy to synthesize the pillar[5]arene-based azobenzene-bridged [3]rotaxanes (Scheme 1). The alkylation of 4, 4′-dihoxylazobenzene with 3-bromoprop-1-yne readily afforded the triple bond bearing photo-active segments. The triple bonds attached to the functional core facilitate subsequently constructing of precursors of guest molecules by Sharpless copper(I)-catalyzed Sharpless alkyne-azide click reaction (Scheme 1). The triazole groups formed in this step could help "fix" the pillar[5]arenes by multiple hydrogen bonds interactions. With the consideration that alkyl chain length on the guest molecule may have different influence on the photo-assisted shuttle property of the supramolecules, we synthesized three azide-end capped segments with varying alkyl chain lengths (C4, C6 and C10). The symmetrical azobenzene bearing threads (5a-5c) was also synthesized as standard. The alkyl chain length did not show obvious influence on the yield of the click reaction. The threading of pillar[5]arene onto the guest molecule was achieved in chloroform solution. And the benzaldehyde end capped pseudo rotaxanes was immediately reacted with 5, 5-dimethylcyclohexane-1, 3-dione and malononitrile to install the bulky stopper to block the encapsulated pillar[5]arenes. In contrast to the situation of 6a and 6b, the yield for 6c was obviously reduced (24% for 6a, 26% for 6b and 8% for 6c), may suggest a relatively low threading efficiency of pillar[5]arene in the case of molecular thread of C10 chain.
|
Download:
|
| Scheme 1. Synthetic route for modes 5a-5c and [3]rotaxanes 6a-6c. | |
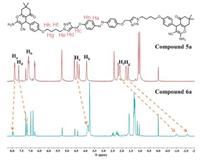
In addition to these [3]rotaxanes, we also synthesized bulky stopper installed guest molecules (5a-5c) without threading of pillar[5]arenes in order to examine the influence of alkyl chain length on photo-responsive property in advance. 1H, 13C NMR and high-resolution mass spectra data for these compounds were shown in Supporting information. As shown in Fig. 1, the protons Hd-Hg of compound 6a exhibited obvious upfield δ movements in contrast to 5a. The upfield δ value of Hd (Δδ = -0.447 for Hd) suggested the formation of C—H···O hydrogen bonds between triazole segment and the oxygen atoms on the pillar[5]arene as documented in the literature [22]. The upfield δ movements of protons He to Hh suggested inclusion of the methylene units within the cavity of pillar[5]arene as the result of the encapsulation-induced shielding effects [23]. These results clearly indicated formation of unique [3]rotaxanes. In addition to the 1H NMR spectra, the exact molecular weight of 6a measured by high-resolution mass matched the calculated value, further indicating the formation of [3]rotaxanes (Figs. S22, S25 and S28 in Supporting information).
|
Download:
|
| Fig. 1. Partial 1H NMR spectra of 5a and 6a (CDCl3, 400 MHz). | |
It should be noticed that the small cavity size of pillar[5]arene (inner diameter of around 4.7 Å) typically does not allow it to encapsulate the azobenzene segment (the diameter of benzene ring is around 5.0 Å). Therefore, it is highly possible that the pillar[5]arene would be squeezed to locate on the alkyl chain segment of guest molecules. After the threading of pillar[5]arenes, δ values of Hf and Hg on the alkyl chain 6a displayed dramatically upfield in movements among all the alkyl chain protons (Δδ = -3.370 and -3.180 for Hf and Hg; Δδ = -0.065 and -0.582 for He and Hh). Such alternation in NMR indicated strong C—H···π interactions between the pillar[5]arenes [24]. On the other hand, the splitted aromatic protons on pillar[5]arene (δ = 6.99 and 6.86 for aromatic protons in 6a suggested different chemical environments of the two rims of pillar[5]arenes upon encapsulation. Based on these 1H NMR results, it was reasonable to predict the pillar[5]arenes located near the triazole segments of the guest molecule with the cavity covering He-g of the alkyl chain. This configuration was further supported by the DFT optimized structures of the compound 5a and 6a, based on which the computed upfielded δ for Hf and Hg is ∼3 ppm, in good agreement with the experimental observation mentioned above (Fig. 2).

|
Download:
|
| Fig. 2. Optimized structures of 5a and 6a in dichloromethane solution (hydrogens Hf and Hg are highlighted in cyan). | |
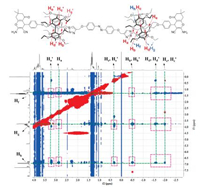
The δ values of alkyl chain protons of the guest molecules of 6b and 6c exhibited similar upshift pattern in contrast to the corresponding model guest compounds. This also indicated the formation of corresponding [3]rotaxanes for the compounds 6b and 6c, and was also supported by high-resolution mass results (Supporting information). Additionally, in NOESY spectra of 6b, the clear NOEs signals have been observed between aromatic (Hh), methylene bridge (Hg) and ethoxyl (Hg and Hf) and the guest alkyl chain protons (Ha to Hd, Fig. 3). This further proved host pillar[5]arenes were encapsulated on the guest molecular thread and the location of the host pillar[5]arene was on the alkyl chain of the guest thread. 1H NMR results revealed more complicated situations of 6b and 6c. Couples of new proton resonance signals were observed in these spectra due to the incorporation of longer alkyl chains in the precursors. As judged from the resonance spectra, the shielding effect of pillar[5]arene was influenced by the alkyl chain length. For instance, in the case of compound 6b, the highest field proton signal of the alkyl chain appeared around -2.02 ppm. However, in compound 6c, the corresponding appeared around -1.51 ppm, whereas, the corresponding protons in 5b and 5c appeared both around 1.90 ppm. In addition, aromatic protons of pillar[5]arene in 6b and 6c exhibited slightly different δ values (corresponding δ for 6b were 6.94 and 6.90; δ for 6c were 6.92 and 6.88). This suggested slightly different shielding effect of pillar[5]arene in synthesized [3]rotaxanes. In compound 6a, proton Hd was found to be merged with Hb in resonance spectrum, in the case of 6b and 6c, these two protons clearly split into two separated peaks (δ for Hd in 6b was 7.63; δ for Hd in 6c was 7.40). This suggested varied C—H···O hydrogen bond situations between Hd and oxygen atom in pillar[5]arene in 6b and 6c in contrast to 6a. The detailed 1H NMR spectra for 6b, 6c and their corresponding 5b and 5c were shown in Figs. S2 and S3 (Supporting information). We attempted to obtain the single crystal of the three compounds to conduct an in-depth analysis of the configurations, yet we failed to obtain the desired single crystals.
|
Download:
|
| Fig. 3. NOESY spectrum of 6b in CDCl3. | |
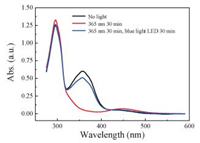
In order to examine the photo-switch properties of [3]rotaxanes 6a, 6b and 6c, we conducted the light irradiation experiments. As shown in Fig. 4, compound 6a exhibited partially photo-switch property as characterized by UV–vis. Upon irradiation with UV light of 365 nm with the light intensity of 2.88 mW/cm2 for 30 min, the absorbance of 6a centered at 350 nm, which corresponds to the π-π* electronic transition of cis-azobenzene isomer, reduced dramatically. This suggested the occurrence of trans to cis under UV irradiation of 6a. Subsequently, irradiating 6a with blue light LED resulted in the 350 nm absorption recovery, meaning occurrence of reverse isomerization [25]. But the absorbance did not completely restore, meaning comprised cis to trans photo-isomerization efficiency. Compounds 6b and 6c displayed similar incomplete reversible transformation upon light irradiation according to the UV–vis spectra (Figs. S3 and S4 in Supporting information).
|
Download:
|
| Fig. 4. UV–vis absorption spectra of 6a (black line); irradiated with 365 nm light (red line) and subsequently irradiated with blue light LED (blue line). Irradiation time was 30 min. | |
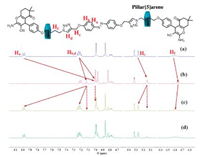
Irradiating 6a with blue light LED for 30 min resulted in partially regain of the chemical shifts for these protons, indicating the location regain of the pillar[5]arenes (Fig. 5). The coexistence of both representative chemical shifts for both isomers proved the incomplete cis/trans isomerization. This is consistent with the result of UV–vis measurement. The detailed cis/trans ratio was determined by comparing the integration of Hc and Hc' to be approximately 77/23. Extended time irradiating experiment (150 min of blue light LED) afforded the identical 1H NMR results as that of 30 min irradiation, indicating the cis to trans photo-isomerization for compound 6a has reached chemical equilibrium regardless of irradiation time and dose. The further irradiation experiment using the end capped guest model molecule 5a displayed nearly completely photo-isomerization in both trans-cis and cis-trans directions as judged by 1H NMR spectra (Fig. S5 in Supporting information). This suggested the installation of pillar[5]arene caused the comprised trans to cis photo-isomerization efficiency.
|
Download:
|
| Fig. 5. Partial 1H NMR spectra of compound 6a upon irradiated with 365 nm for 30 min and blue light LED, a: no irradiation, b: 365 nm for 30 min, c: blue light LED for 30 min, d: blue light LED for 150 min. CD2Cl2, 400 MHz. | |
Irradiation with 365 nm light also resulted in nearly complete trans/cis conversion of compounds 6b and 6c, as proved by disappearance of corresponding Ha from its original position in 1H resonance spectra. In the backwards photo-isomerization of 6b using blue light LED, Hd splitted into two peaks in resonance spectra with δ of 7.63 and 7.58, respectively. The peak of δ 7.63 matched the corresponding peak in 6b without light irradiation. This may suggest a different C–H···O hydrogen bonding situation between Hd and oxygen atom on pillar[5]arenes in cis and trans isomers of 6b. Also, the cis/trans ratio was determined to be 71/29 by comparing the intergation of the split Hd. Corresponding Hd in compound 6c (δ of 7.40 without light irradiation) shifted upfield to 7.32 ppm after UV light irradiation, and partially regained the original chemical shift in 1H NMR resonance spectra. The corresponding cis/trans ratio was calculated to be 71/29 as well. The similar cis/trans ratio of synthesized [3]rotaxanes indicated the longer alkyl chain of the guest molecular thread did not result in improved photo-isomerization efficiency. The results of irradiation experiment using corresponding 5b and 5c resembles the case of 5a, further proved the threading of pillar[5]arene could be the main reason for incompletely trans to cis photo-isomerization (Figs. S7 and S8 in Supporting information).
We subsequently investigated the light-triggered shuttle behaviors of 6b and 6c using 1H NMR. The shielding effects of pillar[5]arene were observed to increase with alkyl chain length (the most prounced upfield δ variations of the alkyl chain protons of 6b and 6c amounted to -0.07 and -0.09, respectively). The enhanced Δδ of alkyl chain of 6b and 6c in contrast to 6a in the process of photo irradiation suggested longer alkyl chain could faciliate the shuttle behavior of pillar[5]arene to a certain extent. However, the photo-triggered shuttle behavior of [3]rotaxanes was believed to be confined to a small extent independent of the alkyl chain lengths. One plausibile reason could be the strong "hydrogen bond locking effect" between the triazole and pillar[5]arenes that resticted the shifting of pillar[5]arenes even in the streically crowed trans form [3]rotaxanes. The partial 1H NMR spectra of compounds 6b and 6c after light irradiation were shown in Figs. S9 and S10 (Supporting information).
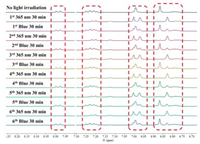
Repeated photo-isomerization of compound 6a can be realized by alternatively irradiation with 365 nm light and blue light LED, respectively. As shown in Fig. 6, the protons in aromatic region of 6a displayed reproducible chemical shift changes within five irradiation cycles. Compounds 6b and 6c also showed repeated photo-isomerization properties as characterized by alternatively irradiation experiments. The partial 1H NMR spectra of 6b and 6c were shown in Figs. S11 and S12 (Supporting information).
|
Download:
|
| Fig. 6. Partial 1H NMR spectra of compound 6a upon irradiated with 365 nm and blue light LED for 30 min for 6 cycles (CD2Cl2, 400 MHz). Protons in the red squares referred to the aromatic protons. | |
To best of our knowledge, we have for the first time constructed series of azobenzene bridged pillar[5]arene-based [3]rotaxanes with different alkyl chain guest molecule (6a, 6b and 6c). 1H NMR and NOESY and high-resolution mass spectra proved the inclusion of the pillar[5]arenes onto the guest molecule, the main driving force for formation of the [3]rotaxanes include C—H···O hydrogen bond and C—H···π interactions. As characterized by UV–vis and 1H NMR spectroscopy, the synthesized rotaxanes could undergo completely trans to cis isomerization under irradiation with 365 nm light for 30 min. Illumination of rotaxanes by blue light did not yield completely backward cis to trans isomerization even in extended irradiation time and intensity. The installation of pillar[5]arene was believed to be the reason for incomplete photo-isomerization. The slightly photo-triggered shuttle behavior of [3]rotaxanes have been observed in the 1H NMR spectra. We are currently focusing on the developing photo responsive [3]rotaxanes with improved shuttle behavior.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21372192, 21871227), the Natural Science Foundation of Jiangsu Province (No. BK20190905) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. Yang Wang acknowledges the Thousand Talents Plan for Young Professionals of China.
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.11.020.
| [1] |
(a) T. Kakuta, T. Yamagishi, T. Ogoshi, Acc. Chem. Res. 51 (2018) 1656-1666; (b) M. Zhang, X. Yan, F. Huang, Z. Niu, H.W. Gibson, Acc. Chem. Res. 47 (2014) 1995-2005. |
| [2] |
K. Miyamae, M. Nakahata, Y. Takashima, et al., Angew. Chem. Int. Ed. 54 (2015) 8984-8987. DOI:10.1002/anie.201502957 |
| [3] |
X.L. Ni, S. Chen, Y. Yang, et al., J. Am. Chem. Soc. 138 (2016) 6177-6183. DOI:10.1021/jacs.6b01223 |
| [4] |
M. Zhang, X. Yan, F. Huang, et al., Acc. Chem. Res. 47 (2014) 1995-2005. DOI:10.1021/ar500046r |
| [5] |
(a) Y. Liu, K. Shi, D. Ma, Chem. Asian J. 14 (2019) 307-312; (b) Y. Sun, J. Ma, F. Zhang, et al., Nat. Commun. 8 (2017) 260; (c) D. Xia, G. Yu, J. Li, et al., Chem. Commun. 50 (2014) 3606-3608; (d) G. Yu, C. Han, Z. Zhang, et al., J. Am. Chem. Soc. 134 (2012) 8711-8717. |
| [6] |
(a) P. Li, Y. Chen, Y. Liu, Chin. Chem. Lett. 30 (2019) 1190-1197; (b) Q. Zhang, D.H. Qu, H. Tian, Adv. Opt. Mater. 7 (2019) 1900033; (c) Y. Zhou, K. Jie, F. Huang, Chem. Commun. 54 (2018) 12856-12859; (d)Y.M. Zhang, N.Y. Zhang, K. Xiao, et al., Angew. Chem. Int. Ed. 57 (2018) 8649-8653. |
| [7] |
D.H. Qu, Q.C. Wang, Q.W. Zhang, et al., Chem. Rev. 115 (2015) 7543-7588. DOI:10.1021/cr5006342 |
| [8] |
M. Xue, Y. Yang, X. Chi, et al., Chem. Rev. 115 (2015) 7398-7501. DOI:10.1021/cr5005869 |
| [9] |
G. Yu, X. Zhou, Z. Zhang, et al., J. Am. Chem. Soc. 134 (2012) 19489-19497. DOI:10.1021/ja3099905 |
| [10] |
R. Zhang, X. Yan, H. Gao, et al., Chem. Commun. 56 (2020) 948-951. DOI:10.1039/C9CC09155F |
| [11] |
(a) M. Wolf, A. Ogawa, M. Bechtold, et al., Chem. Sci. 10 (2019) 3846-3853; (b) X. Yao, T. Li, J. Wang, et al., Adv. Opt. Mater. 4 (2016) 1322-1349. |
| [12] |
H.Y. Zhou, Y. Han, Q. Shi, et al., Eur. J. Org. Chem. 21 (2019) 3406-3411. |
| [13] |
G. Baggi, L. Casimiro, M. Baroncini, et al., Chem. Sci. 10 (2019) 5104-5113. DOI:10.1039/C9SC00913B |
| [14] |
A. Saura-Sanmartin, A. Martinez-Cuezva, A. Pastor, et al., Org. Biomol. Chem. 16 (2018) 6980-6987. DOI:10.1039/C8OB02234H |
| [15] |
(a) G. Yu, C. Han, Z. Zhang, et al., J. Am. Chem. Soc. 134 (2012) 8711-8717; (b) Y. Chen, S. Sun, Y. Yao, et al., Chin. Chem. Lett. 30 (2019) 37-43; (c) R. Zhang, C. Wang, Y. Yao, et al., Front. Chem. 7 (2019) 508; (d) S. Sun, T. Liu, Y. Yao, et al., J. Colloid. Interf. Sci. 533 (2019) 42-46. |
| [16] |
(a) K. Iwaso, Y. Takashima, A. Harada, Nat. Chem. 8 (2016) 626-633; (b) Y. Han, C.Y. Nie, S. Jiang, et al., Chin. Chem. Lett. 31 (2020) 725-728; (c) J. Ye, R. Zhang, W. Yang, et al., Chin. Chem. Lett. 31 (2020) 1550-1553; (d) R. Zhang, C. Wang, J. Sun, et al., Chin. J. Org. Chem. 39 (2019) 3483-3489; (e) L.L. Zhao, Y. Han, C.G. Yan, Chin. Chem. Lett. 31 (2020) 81-83. |
| [17] |
Z. Yang, X. Liu, S. Zhao, J. He, Prog. Chem. 26 (2014) 1899-1913. |
| [18] |
(a) X. Chi, X. Ji, D. Xia, et al., J. Am. Chem. Soc. 137 (2015) 1440-1443; (b) X.Y. Hu, K. Jia, Y. Cao, et al., Chem. Eur. J. 21 (2015) 1208-1220; (c) H. Zhang, N.L. Strutt, R.S. Stoll, et al., Chem. Commun. 47 (2011) 11420-11422; (d) T. Ogoshi, D. Yamafuji, T. Aoki, et al., J. Org. Chem. 76 (2011) 9497-9503. |
| [19] |
(a) T. Xiao, L. Zhou, L. Xu, et al., Chin. Chem. Lett. 30 (2019) 271-276; (b) T. Xiao, W. Zhong, L. Zhou, et al., Chin. Chem. Lett. 30 (2019) 31-36; (c) C. Ke, N.L. Strutt, H. Li, et al., J. Am. Chem. Soc. 135 (2013) 17019-17030. |
| [20] |
(a) T. Xiao, L. Qi, W. Zhong, et al., Mater. Chem. Front. 3 (2019) 1973-1993; (b) D. Cao, H. Meier, Isr. J. Chem. 58 (2018) 1188-1193; (c) S. Pan, M. Ni, B. Mu, et al., Adv. Funct. Mater. 25 (2015) 3571-3580. |
| [21] |
H. Zhang, Z. Liu, Y. Zhao, Chem. Soc. Rev. 47 (2018) 5491-5528. DOI:10.1039/C8CS00037A |
| [22] |
S. Wang, X. Shao, W. Cai, J. Phys. Chem. C 121 (2017) 25547-25553. DOI:10.1021/acs.jpcc.7b07279 |
| [23] |
X. Shu, S. Chen, J. Li, et al., Chem. Commun. 48 (2012) 2967-2969. DOI:10.1039/c2cc00153e |
| [24] |
H. Murakami, A. Kawabuchi, R. Matsumoto, et al., J. Am. Chem. Soc. 127 (2005) 15891-15899. DOI:10.1021/ja053690l |
| [25] |
T. Ogoshi, K. Yoshikoshi, T. Aoki, et al., Chem. Commun. 49 (2013) 8785-8787. DOI:10.1039/c3cc44384a |
 2021, Vol. 32
2021, Vol. 32 

