b Department of Chemistry, University of California Irvine, CA 92697, United States
Since E. Yablonovitch [1] and S. John [2] independently proposed the concept of photonic crystals (PhCs) in 1987, PhCs have attracted more and more attentions. PhC is a material consisting of a periodic arrangement of 1D, 2D, and 3D nanostructures with different refractive indices [3]. Based on their unique optical properties, many sensing applications such as temperature [4], humidity [5], pH [6, 7], organic solvents [8], glucose [9] and metal ions [10-12] have been explored.
Most of colloidal PhC sensors composed of synthetic hydrogels matrix [13], especially N-isopropylacrylamide (NIPAM) hydrogel [14] and HEMA-co-AAc hydrogel [15]. The chemically crosslinked hydrogels need harsh reaction conditions and the reactants and products are usually toxic. For the above reasons, biomaterials have received extensive attention for their biocompatibility, ease of preparation, non-toxicity, and environmental friendliness. For example, cellulose which is widely found in plants and bacteria, has been combined with PhCs to exhibit good response to various organic solvents [16] and vapors [17]. PhC sensors [15, 18] based on silk protein extracted from silkworm cocoons have shown a good response to humidity [19, 20]. Butterfly wings, a natural PhC composed of chitin, have been developed into a variety of PhC sensors that respond well to organic vapors [21] and stress [22]. However, biomass materials are generally poor in water resistance and mechanical strength.
As a traditional Chinese food, "konjac tofu" is an edible gel. Its main component, konjac glucomannan (KGM) undergoes selfentanglement and self-aggregation after deacetylation in an alkaline environment, and a 3D network formed by hydrogen bonding [23]. KGM is a water-soluble linear polysaccharide isolated from the konjac tuber [24]. Its main chain is formed by the linkage of D-mannose and D-glucose through an α-1, 4-glycosidic bond. In addition, about 19 saccharide residues are attached to the side chain of the acetyl group [25], which can only be hydrolyzed by the human intestinal and colonic terminal mannose hydrolase [26]. KGM has been authorized as a food additive in Europe and has been classified by FDA (Food and Drug Administration, United States) as GRAS (generally considered safe) [27]. Because of its outstanding film forming and gelation ability, besides food industry [28], it has also received extensive attention from the fields of pharmaceutical carriers [29], tissue scaffolds [30], aerogel [31] and self-healing material [32].
We introduce the concept of double network (DN) hydrogel to improve the strength of KGM self-crosslinked hydrogel. DN hydrogel is a special interpenetrating polymer networks (IPN) hydrogel [33, 34] consisting of two polymeric networks with strong asymmetric structures [35], which is a combination of a hard and brittle first network and a flexible second network consisting of two polymer networks with strong asymmetric structures [36]. The double network strategy is widely used in polysaccharidebased hydrogels to increase its strength, such as cellulose gel [34], alginate gel [37], carrageenan gel [38], gelatin gel [39] etc. In addition, KGM DN gel has also attracted academic attentions. Li et al. [40] used polyvinyl alcohol (PVA) as a macro-crosslinking agent to prepare a high toughness DN hydrogel of KGM and polyacrylamide (PAAm), and showed excellent cell adhesion properties.
DN gel is not only used to improve mechanical strength, biocompatibility and other properties, but also has received wide attention in the fields of surface modification. Taking advantage of the polysaccharide's natural hydrophilicity, a large number of polysaccharide-based DN gels were prepared in order to improve the surface wettability. For example, S. Jalali et al. [41] used Tragacanth Gum (TG) to improve the hydrophilicity and surface wettability of polyethylene terephthalate. C. Lin et al. [42] used chitosan to effectively reduce the contact angle of silicone hydrogels. J. Dutra et al. [43] improved the wettability of the polyvinyl alcohol/calcium alginate composite film by adjusting the calcium alginate concentration. J. Zhang et al. [44] used Kappa and carrageenan to form a semi-interpenetrating structure to effectively improve the surface wettability of the gel. In summary, DN gel has greatly expanded the application of polysaccharide gel in the fields of biomedical materials, smart materials and wearable devices.
Herein, based on the work of Li et al. [40], We developed a more concise synthesis strategy to prepare a new polysaccharide-based DN hydrogel. PVA was introduced into the hydrogel system as a hard skeleton, which not only maintained the biocompatibility of konjac glucomannan, but also gave the hydrogel superior mechanical properties and solvent resistance. By combining hydrogels with PhCs, we obtained a KGM-based PhC sensor that responds well to a variety of physical and chemical stimulus, especially for methanol.
This sensor responded to methanol and methanol vapor, exhibited a good linear relationship, and had the ability to detect methanol in gasoline, which has a good application prospect.
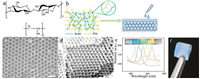
Both KGM and PVA are rich in hydroxyl groups (Fig. 1a), therefore, we cross-linked KGM and PVA with glutaraldehyde under acidic conditions to form a double network hydrogel (Fig. 1b) with a clear Tyndall effect (Fig. S1 in Supporting information). Then we infiltrated the hydrogel into a 3D PMMA array (Fig. S2 in Supporting information) to obtain an opal film. It could be observed from scanning electron microscopy (SEM) (Fig. 1c, Fig. S3 in Supporting information) that the periodic structure of the 3D PMMA array was completely preserved in the gel. In addition, after etching the PMMA colloidal by toluene, we obtained a highly regular inverse opal structure (Fig. 1d), and the nanopores were connected to each other. Five opal PhC films and inverse opal PhC films were prepared using the methods in Supporting information, and the wavelengths of their reflection peaks were measured by fiber optic spectrometer. The results are shown in Fig. 1e, and the digital images are the structural colors of the PhC films corresponding to the respective reflection peaks. The PhC films in the first and second pictures of the left are inverse opal structure, and the remaining PhC films are opal structure. All of the PhC films exhibit strong reflection peaks and bright structural colors, which also prove that the highly periodic ordered structure of the PhC is completely preserved in the gel film. In Fig. 1f, it can be observed that the finally obtained KGM PhCs film has a bright structural color, and could be bent and folded in the completely dry state, exhibit good mechanical strength and flexibility and far surpass the reported cellulose PhC film. In fact, after been immersed into water, the stress-strain curve (Fig. S4 in Supporting information) of the film shows its superior tensile properties, and the maximum strain of the hydrogel film is 168%. The difference from the conventional hydrogel film is that this KGM PhCs film can be dried and stored at a normal temperature for a long period of time without been immersed in water for storage.
|
Download:
|
| Fig. 1. (a) Chemical structure of KGM and PVA. (b) Schematic of double network formed by KGM and PVA cross-linking with glutaraldehyde, and hydrogel combined with PhCs. (c) SEM images of KGM-PVA 3D photonic crystal films. (d) SEM images of inverse opal photonic crystal film. (e) The spectrum of the opal photonic crystal film (3, 4, 5) and the inverse opal photonic crystal film (1, 2), and the inserted photographs are the structural colors of films. (f) Photograph of KGM-PVA inverse opal photonic crystal films with great flexibility. | |
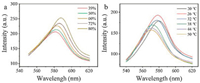
If the KGM PhCs film was stored under different humidity (Fig. S6 in Supporting information), the reflection peak red-shifted as the humidity increase (Fig. 2a). The reason is that KGM is super in water absorption, the film would swell in water in a high humidity environment, causing the lattice spacing to expand, which in turn causes the reflection peak to shift red. In addition to the response to humidity, we also investigated the response of KGM PhCs film to temperature (Fig. 2b, Fig. S5 in Supporting information). When temperature increases, the hydrogel film is dehydrated, the lattice spacing decrease, and the reflection peak shifts to blue.
|
Download:
|
| Fig. 2. Response of photonic crystal film to humidity (a) and temperature (b). | |
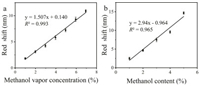
After investigation of the response of the KGM PhCs film to physical stimuli, we found that it also responds well to pure organic solvents such as isopropanol (Fig. 3a), n-propanol (Fig. 3b), n-butanol (Fig. 3c) and petroleum (Fig. 3d) ether, especially alcohols. We chose methanol for further research. As the methanol content in the water increased, the reflection peak of the KGM PhCs red shifted significantly, and the methanol fraction showed potential linear correlation with the peak wavelength of the reflection (Fig. 4a). After regenerating the film for 6 rounds, it still maintained its sensing performance, indicating excellent stability (Fig. 4b).

|
Download:
|
| Fig. 3. Response of photonic crystal film to isopropanol (a), n-propanol (b), n-butanal (c), petroleum ether (d). | |

|
Download:
|
| Fig. 4. (a) Response of photonic crystal film to methanol content in water. (b) The recovery of photonic crystal film over five cycles. (c) Response of photonic crystal film to water content in methanol. (d) Linear relationship between red shift of reflection peak of photonic crystal film and water content in methanol. Each error bar is obtained by repeating more than three experiments on one chip. | |
As we all know, many chemical reactions need to be performed in a strictly anhydrous environment. Therefore, there is an urgent need for monitoring the water content in the reaction system. Our group has long been devoted to the development of sensors in this field. And our team has been conducting related research for a long time. For example, D. Yan et al. [45] developed a silk protein-based sensor for the detection of water content in acetone. In order to develop the application of KGM PhCs film in reaction system monitoring, we introduced a small amount of water in methanol and found that the KGM PhCs film is very sensitive to the change of water fraction in methanol. As the water fraction increased, the reflection peak red shifted, and the structural color gradually changed from green to yellow-green (Fig. 4c), which also showed a good linear relationship (Fig. 4d). In addition, we also detected the methanol vapor by the KGM PhCs film. We built a sealed device, as shown in Fig. S7 (Supporting information). We used a gas syringe to inject trace amount of methanol through a gas valve and fully volatilized to produce methanol vapor. The relationship between the methanol liquid volume and the vapor concentration could be calculated according to the following formula (Eq. 1):
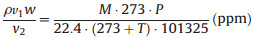
|
(1) |
where p is the methanol density (g/m3), v1 is the volume of injected methanol (μL), v2 is the volume of the container (mL), w is the purity of methanol (%), M is the relative molecular mass of methanol (g/mol), T is the absolute temperature (K), P is the atmospheric pressure (Pa). According to the above formula, the methanol concentration in ppm can be obtained. Since the methanol concentration in our experiment is large, we convert ppm to percent (%), and the results are shown in Fig. 5a. With the adsorption of methanol vapor by films, the reflection peak redshifts. It is worth mentioning that this process occurs very quickly. Based on the results in Fig. S8 (Supporting information), we randomly selected two films, and they both responded to methanol vapor within 1-2 min. For different concentrations of methanol vapor, the PhC film has different degrees of redshift (Fig. S9 in Supporting information). And the amount of red shift correlates linearly (R2 = 0.993) to methanol vapor concentration (Fig. 5a).
|
Download:
|
| Fig. 5. (a) Linear relationship between red shift of reflection peak of photonic crystal film and methanol vapor concentration. (b) Linear relationship between red shift of reflection peak of photonic crystal film and methanol content in gasoline. Each error bar is obtained by repeating more than three experiments on one chip. | |
In order to verify the ability of the KGM PhCs film to detect the methanol content in real sample, we applied the film for the fast screening of methanol fraction in gasoline. There are unscrupulous traders who add methanol to gasoline for benefits. However, traditional detection methods required sophisticated and expensive chromatography instruments, which take a long time and are difficult to be use for on-site screening. Therefore, we introduced the KGMPhCsfilmfor the rapid screening of methanol in No.92 gasoline. According to Fig. 5b, we can see that as the methanol content in gasoline increases, the reflection peak of the KGM PhCs film is redshifted (Fig. S10 in Supporting information) and shows a good linear relationship, the lowest concentration that has been actually measured is 1%. It can be proved that in complicated samples, the KGM PhCs film still has good detection ability for methanol.
In summary, we prepared an interpenetrating polymer network hydrogel of konjac glucomannan and polyvinyl alcohol by crosslinking with glutaraldehyde. This biomass composite overcomes the problems of poor biocompatibility, easy degradation and harsh synthetic conditions of traditional chemically synthesized hydrogels. The interpenetrating network inside the KGM-PVA hydrogels make it show outstanding mechanical strength and water resistance. By implanting the KGM hydrogel combined with opal structure, a new type of biomass based photonic crystal sensor was obtained, which has good response to various organic solvents and temperature and humidity. Among them, the response to alcohol is the most prominent. Not only does it exhibit a good response in methanol-water solution, but it also exhibits a good detection ability in addition to methanol vapor, and both have a stable and significant linear relationship. In addition, it can show fast responses and great linear relationship to methanol in No. 92 gasoline, and the lowest concentration that has been actually measured is 1%. Based on KGM PhCs film, an on-site methanol screening method for the quality control of gasoline is expected.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis research was supported by the National Natural Science Foundation of China (Nos. 21874009, 21804009).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.012.
| [1] |
E. Yablonovitch, Phys. Rev. Lett. 58 (1987) 2059-2062. DOI:10.1103/PhysRevLett.58.2059 |
| [2] |
S. John, Phys. Rev. Lett. 58 (1987) 2486-2489. DOI:10.1103/PhysRevLett.58.2486 |
| [3] |
E.P.A. van Heeswijk, A.J.J. Kragt, N. Grossiord, A.P.H.J. Schenning, Chem. Comm. 55 (2019) 2880-2891. DOI:10.1039/C8CC09672D |
| [4] |
M. Moirangthem, T.A.P. Engels, J. Murphy, C.W.M. Bastiaansen, A.P.H.J. Schenning, ACS Appl. Mater. Interfaces 9 (2017) 32161-32167. DOI:10.1021/acsami.7b10198 |
| [5] |
M. Moirangthem, A.P.H.J. Schenning, ACS Appl. Mater. Interfaces 10 (2018) 4168-4172. DOI:10.1021/acsami.7b17892 |
| [6] |
A.K. Yetisen, H. Butt, F. da Cruz Vasconcellos, et al., Adv. Optical Mater. 2 (2014) 250-254. DOI:10.1002/adom.201300375 |
| [7] |
D. Shi, X. Zhang, Z. Yang, S. Liu, M. Chen, RSC Adv. 6 (2016) 85885-85890. DOI:10.1039/C6RA18738B |
| [8] |
A.K. Yetisen, M.M. Qasim, S. Nosheen, T.D. Wilkinson, C.R. Lowe, J. Mater. Chem. C 2 (2014) 3569-3576. DOI:10.1039/c3tc32507e |
| [9] |
C. Zhang, G.G. Cano, P.V. Braun, Adv. Mater. 26 (2014) 5678-5683. DOI:10.1002/adma.201401710 |
| [10] |
W. Hong, H. Li, X. Hu, et al., Chem. Comm. 48 (2012) 4609-4611. DOI:10.1039/c2cc30927k |
| [11] |
H. Saito, Y. Takeoka, M. Watanabe, Chem. Comm. 3 (2003) 2126-2127. |
| [12] |
A.K. Yetisen, Y. Montelongo, M.M. Qasim, et al., Ana. Chem. 87 (2015) 5101-5108. DOI:10.1021/ac504274q |
| [13] |
A.K. Yetisen, H. Butt, L.R. Volpatti, et al., Biotechnol. Adv. 34 (2016) 250-271. DOI:10.1016/j.biotechadv.2015.10.005 |
| [14] |
M. Chen, L. Zhou, Y. Guan, Y. Zhang, Angew. Chem. Int. Ed. 52 (2013) 9961-9965. DOI:10.1002/anie.201302466 |
| [15] |
M. Qin, M. Sun, R. Bai, et al., Adv. Mater. 30 (2018) 1-7. |
| [16] |
F. Wang, Z. Zhu, M. Xue, et al., Sens. Actuator. B: Chem. 220 (2015) 222-226. DOI:10.1016/j.snb.2015.05.057 |
| [17] |
P. Lova, Polymers 10 (2018) 1161. DOI:10.3390/polym10101161 |
| [18] |
B. Marelli, F.G. Omenetto, J. Mater. Chem. C 3 (2015) 2783-2787. DOI:10.1039/C5TC00056D |
| [19] |
Y.Y. Diao, X.Y. Liu, G.W. Toh, L. Shi, J. Zi, Adv. Funct. Mater. 23 (2013) 5373-5380. DOI:10.1002/adfm.201203672 |
| [20] |
Y. Wang, M. Li, E. Colusso, W. Li, F.G. Omenetto, Adv. Opt. Mater. 6 (2018) 1-7. |
| [21] |
G. Piszter, K. Kertész, Z. Bálint, L.P. Biró, Sensors 16 (2016) 1-916. DOI:10.1109/JSEN.2016.2616227 |
| [22] |
Z. Chen, F. Fu, Y. Yu, et al., Adv. Mater. 31 (2019) 1-7. |
| [23] |
X. Luo, P. He, X. Lin, Food Hydrocoll. 30 (2013) 92-99. DOI:10.1016/j.foodhyd.2012.05.012 |
| [24] |
W. Fang, P. Wu, Food Hydrocoll. 18 (2004) 167-170. DOI:10.1016/S0268-005X(03)00044-4 |
| [25] |
L. Huang, R. Takahashi, S. Kobayashi, T. Kawase, K. Nishinari, Biomacromolecules 3 (2002) 1296-1303. DOI:10.1021/bm0255995 |
| [26] |
S.S. Behera, R.C. Ray, Food Rev. Int. 33 (2017) 22-43. DOI:10.1080/87559129.2015.1137310 |
| [27] |
F. Jimenez-Colmenero, S. Cofrades, A.M. Herrero, M.T. Solas, C. Ruiz-Capillas, Food Hydrocoll. 30 (2013) 351-357. DOI:10.1016/j.foodhyd.2012.06.015 |
| [28] |
D.F. da Silva, S.B. de, S. Ferreira, M.L. Bruschi, M. Britten, P.T. Matumoto-Pintro, Food Hydrocoll. 60 (2016) 308-316. DOI:10.1016/j.foodhyd.2016.03.034 |
| [29] |
M. Lu, Z. Li, H. Liang, et al., Food Hydrocoll. 51 (2015) 476-485. DOI:10.1016/j.foodhyd.2015.05.036 |
| [30] |
L. Fan, J. Yi, J. Tong, et al., Int. J. Biol. Macromolecules 91 (2016) 358-367. DOI:10.1016/j.ijbiomac.2016.05.042 |
| [31] |
Y. Si, X. Wang, C. Yan, et al., Adv. Mater. 28 (2016) 9512-9518. DOI:10.1002/adma.201603143 |
| [32] |
Z. Li, Y. Su, M.A. Haq, B. Xie, D. Wang, Polymer 103 (2016) 146-151. DOI:10.1016/j.polymer.2016.09.046 |
| [33] |
A. Kausar, Polym.-Plast. Technol. Mater. 58 (2019) 691-706. |
| [34] |
A. Nakayama, A. Kakugo, J.P. Gong, et al., Adv. Funct. Mater. 14 (2004) 1124-1128. DOI:10.1002/adfm.200305197 |
| [35] |
J.P. Gong, Y. Katsuyama, T. Kurokawa, Y. Osada, Adv. Mater. 15 (2003) 1155-1158. DOI:10.1002/adma.200304907 |
| [36] |
Y. Liu, W. He, Z. Zhang, B. Lee, Gels 4 (2018) 46. DOI:10.3390/gels4020046 |
| [37] |
J.Y. Sun, X. Zhao, W.R.K. Illeperuma, et al., Nature 489 (2012) 133-136. DOI:10.1038/nature11409 |
| [38] |
X. Lu, C.Y. Chan, K.I. Lee, et al., J. Mater. Chem. B 2 (2014) 7631-7638. DOI:10.1039/C4TB01289E |
| [39] |
D.M. Kirchmajer, M. Panhuis, J. Mater. Chem. B 2 (2014) 4694-4702. DOI:10.1039/C4TB00258J |
| [40] |
Z. Li, Y. Su, B. Xie, et al., J. Mater. Chem. B 3 (2015) 1769-1778. |
| [41] |
S. Jalali, M. Montazer, R.M.A. Malek, Fiber. Polym. 19 (2018) 2088-2096. DOI:10.1007/s12221-018-8276-y |
| [42] |
C.H. Lin, H.L. Cho, Y.H. Yeh, M.C. Yang, Colloid Surf. B-Biointerfaces 136 (2015) 735-743. DOI:10.1016/j.colsurfb.2015.10.006 |
| [43] |
J.A.P. Dutra, S.G. Carvalho, A.C.D. Zampirolli, et al., Eur. J. Pharm. Biopharm. 113 (2017) 11-23. DOI:10.1016/j.ejpb.2016.12.001 |
| [44] |
J. Zhang, W. Ji, T. Liu, C. Feng, Macromol. Chem. Phys. 217 (2016) 1197-1204. DOI:10.1002/macp.201500517 |
| [45] |
D. Yan, L. Qiu, K.J. Shea, Z. Meng, M. Xue, ACS Appl. Mater. Interfaces 11 (2019) 39163-39170. DOI:10.1021/acsami.9b11576 |
 2021, Vol. 32
2021, Vol. 32 

