b University of Chinese Academy of Sciences, Beijing 100049, China;
c Shanghai Research Institute of Fragrance and Flavor Industry, Shanghai 200232, China;
d School of Perfume and Aroma Technology, Shanghai Institute of Technology, Shanghai 200233, China
Fragrances are widely used in our daily lives [1-4]. Among the most commonly used and most used fragrances in the world each year, linalool is ranked first almost every year. In addition, linalool has the effects of regulating the central nervous system, such as hypnosis, sedation, anticonvulsant and lowering body temperature. However, the aroma in traditional aromatic products is released too quickly [5-7]. On the one hand, this leads to excessive aroma, and further leads to poor aroma experience. On the other hand, this also leads to a shorter service life of aromatic product. Therefore, slowing the release rate of aroma and increasing the fragrance retention time of aromatic products are the key scientific problems that need to be solved at this stage.
There are many ways to achieve sustained release of fragrance molecules, such as metal organic frameworks, 2D materials, and so on. Besides, mesoporous silica nanoparticles are used to adsorb fragrances [8-10]. Since mesoporous silica nanoparticles have large specific surface areas, the release of aroma is delayed. However, mesoporous silica nanoparticles are not easily decomposed and may cause damage to the body after inhalation. The fragrance molecules can also be attached to the polymer or nanoparticle by covalent bonding [11-14]. The sustained release and controlled release of the fragrance molecules are achieved by the breakage of the chemical bond. However, most fragrance molecules do not have groups that can be covalently attached. In other words, this approach is not universal. Amphiphilic block polymers can be used to encapsulate fragrance by forming micelles or liposomes [15-18]. However, the hydrophilic blocks of most amphiphilic block polymers are linear polymer polyethylene glycol (PEG), which cannot protect the fragrance molecules from being released. Therefore, such polymers cannot significantly increase the fragrance retention time of aromatic products.
In this study, zwitterionic comb-like lipid polymers were synthesized to encapsulate linalool in order to improve the encapsulation efficiency of the fragrance and achieve a better sustained release effect. Their hydrophilic blocks were comb-like polymers polycarboxybetaine (PCB), and the hydrophobic blocks were lipid molecules distearoyl phosphoethanolamine (DSPE). Due to the hydrophilic-hydrophobic interaction, the amphiphilic block copolymer DSPE-PCB formed micelles in water. The hydrophilic block PCB was in contact with water on the outside of the micelle, while the hydrophobic block formed a core on the inside of the micelle and encapsulates the hydrophobic linalool.Compared with the linear hydrophilic block PEG, the combshaped hydrophilic block had a large number of side chains. These side chains not only helped DSPE-PCB form denser micelles, they also protected the perfume molecules from being released like fences. Therefore, DSPE-PCB was expected to significantly increase the encapsulation efficiency and limit the rate of release of the fragrance.
2-(N, N'-Dimethylamino)ethyl methacrylate (DMAEMA, 98%), 2-bromoisobutyryl bromide (97%) were obtained from Alfa Aesar. β-Propiolactone (98%) was purchased from J&K Scientific Ltd. 1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] (DSPE-PEG 2000) were purchased from Shanghai Advanced Vehicle Technology Ltd. Linalool was obtained from Shanghai research institute of fragrance. All other chemicals such as ethanol and tetrahydrofuran were analytical grade reagents and were obtained locally.
DSPE-PCB was synthesized according to the method reported by our group using an atom transfer radical polymerization (ATRP) [19]. The 1H NMR spectra was recorded on a Bruker 600 MHz spectrometer. The degree of polymerization and the structure of DSPE-PCB were confirmed by 1H NMR spectra. 1H NMR (CDCl3): δ 1.25 (—(CH2)14—CH3); 1.80 (—NHCOCCH3); 1.00 (—BrCH2CCH3); 4.10 (—OCH2CH2N—); 3.3-3.8 (—OCH2CH2 NCH2CH2—); 2.25 (—NCH3CH3—); 3.3-3.8 (—OOCCH2CH2N—); 2.60 (—OOCCH2CH2N—).
Cationic liposomes were prepared by thin lipid film method. DSPE-PCB20 (10 mg/mL) and linalool (5 mg/mL) at a molar ratio of 1.5:1 were dissolved in chloroform. And DSPE-PEG 2000 (10 mg/mL) and Linalool (5 mg/mL) at a molar ratio of 1.5:1 were dissolved in chloroform. Then the organic phase was removed at 24 ℃ on a rotary evaporator to obtain a thin lipid film. After, the lipid film was hydrated with 2 mL of phosphate buffer saline (PBS) and sonication at 24 ℃ for 30 min. Finally, the obtained sample was vacuum dried for 3 h to obtain linalool@DSPE-PCB and linalool@DSPE-PEG.
The particle size of the above sample in three replicates was determined by a dynamic light scattering using a Malvern Zetasizer nano ZS apparatus (Malvern Instruments, Malvern, United Kingdom). Each sample was measured by a solid state He– Ne laser of 633.0 nm at 25 ℃ with an angle detection of 90°. The size distribution was evaluated for all samples according to the polymer dispersibility index (PDI). PDI is an indicator of the nanocapsule size distribution in the range 0–1. PDI values of < 0.2 indicate a monodisperse emulsion, whereas values > 0.5 show larger distributions.
The content and release of linalool was measured by high performance liquid chromatography (HPLC). For HPLC analysis, we used a mobile phase with water/acetonitrile (v/v = 40%/60%) at a flow rate of 1.0 mL/min and a C18 reversed-phase column (250 × 4.6 × 5 μm). The concentrations of linalool based on the peak area at the retention time of 5 min at 210 nm. The release rate was calculated via the below formula:

|
where W1 was the weight of released linalool and W2 was the total weight of linalool.
The synthetic route of DSPE-PCB20 is shown in Fig. 1A. The monomers CB were synthesized by an addition reaction of β-propiolactone and DMAEMA. Polymers DSPE-PCB20 were synthesized by an atom transfer radical polymerization (ATRP). DSPEBr was initiators, CB was monomers and CuBr/PMDETA were catalysts. As shown in Fig. 1B, 1H HMR proved that DSPE-PCB20 were synthesized successfully. According to the area of characteristic peaks of DSPE (δ 1.25 (—(CH2)14—CH3)) and CB (δ 4.10 (—OCH2CH2N—)), the polymerization degree of DSPE-PCB was calculated as 20.

|
Download:
|
| Fig. 1. (A) The synthetic route of DSPE-PCB. (B) The 1H NMR spectrum of DSPE-PEG. | |
In the following, linalool was encapsulated into DSPE-PCB20. Since DSPE-PCB was amphiphilic block copolymer, they formed micelles in water. The hydrophilic blocks formed micelle shells and the hydrophobic blocks formed micellar cores. Hydrophobic linalool was encapsulated in the cores of the micelles by hydrophobic interaction. The concentration of the amphiphilic block polymers was very important for the formation of micelles. Therefore, four concentrations of DSPE-PCB were chosen to encapsulate linalool. As shown in Fig. 2A, the linalool@DSPEPCB had diameters between 50 nm and 150 nm at different DSPEPCB concentrations. Among them, when the concentration of DSPE-PCB was 10 mg/mL, the diameter of linalool@DSPE-PCB was the smallest, which was 57.31 nm. As shown in Fig. 2B, at different DSPE-PCB concentrations, the linalool@DSPE-PCB had PDI values between 0.15 and 0.25. Among them, when the concentration of DSPE-PCB was 10 mg/mL, the PDI value of linalool@DSPE-PCB was the minimal, which was 0.153. This result indicated that linalool@DSPE-PCB had the most uniform size and the most stable performance when the concentration of DSPE-PCB was 10 mg/mL. Therefore, DSPE-PCB with a concentration of 10 mg/mL were screened to encapsulate linalool.

|
Download:
|
| Fig. 2. (A) The hydrodynamic diameter of linalool@DSPE-PCB with different concentration of DSPE-PCB20. (B) The PDI values of linalool@DSPE-PCB with different concentration of DSPE-PEG20. (C) The TEM image of linalool@DSPE-PCB. (D) The TEM image of linalool@DSPE-PEG2000. The scale bars were 200 nm. | |
The morphologies of linalool@DSPE-PCB and linalool@DSPEPEG were characterized by transmission electron microscopy (TEM). They were negatively stained for TEM detection due to the low contrast of linalool@DSPE-PCB and linalool@DSPE-PEG. As shown in Figs. 2C and D, both linalool@DSPE-PCB and linalool@DSPE-PEG were nearly spherical. The size of linalool@DSPEPEG was smaller than the size of linalool@DSPE-PCB. However, there were many small white spots in the TEM image of linalool@DSPE-PEG. These could be unencapsulated or leaked linalool, or it could be DSPE-PEG2000 that was not involved in selfassembly.
Subsequently, the encapsulation efficiency of linalool@DSPEPCB was detected by HPLC. In contrast, the encapsulation efficiency of linalool@DSPE-PEG was also tested. As shown in Fig. 3, the encapsulation efficiency of linalool@DSPE-PCB reached 1.54 times the encapsulation efficiency of linalool@DSPE-PEG, which was 34.5%. This might be due to that the DSPE-PCB were comb polymers that could form denser micelles. Therefore, linalool@DSPE-PCB can greatly reduce the leakage of fragrance molecules in the process of encapsulating linalool, thereby increasing the encapsulation efficiency of linalool@DSPE-PCB.

|
Download:
|
| Fig. 3. The encapsulation efficiency of linalool@DSPE-PCB and linalool@DSPE-PEG. | |
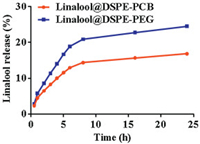
Finally, the release of linalool was detected by HPLC. As shown in Fig. 4, linalool was released much more slowly from linalool@DSPE-PCB compared with linalool@DSPE-PEG. After 24 h, 24.49% linalool was released from linalool@DSPE-PEG. In contrast, only 16.83% of linalool was released from linalool@DSPE-PCB. This might be due to the fact that the DSPE-PCB were comb polymers that could form denser nanoparticles. In addition, the side chain on the comb polymer acted like fences, preventing the release of fragrance molecules from the nanoparticles. Therefore, the fragrance encapsulated by the zwitterionic comb-like lipid polymers DSPE-PCB exhibited a better sustained release effect.
|
Download:
|
| Fig. 4. The cumulative release profiles of linalool from linalool@DSPE-PCB and linalool@DSPE-PEG. | |
In summary, zwitterionic comb-like lipid polymers DSPE-PCB20 were synthesized to encapsulate linalool and named linalool@DSPE-PCB. The hydrodynamic diameter was 57.31 nm and the PDI value was minimal when the concentration of DSPE-PCB was 10 mg/mL. DSPE-PCB significantly increased the encapsulation efficiency of linalool compared to DSPE-PEG. In addition, these zwitterionic comb-like lipid polymers also significantly slowed the release rate of fragrance and prolonged the fragrance retention time of nano-fragrance compared to linear polymers DSPE-PEG. Therefore, this nano-fragrance based on zwitterionic comb-like lipid polymers was expected to be an ideal aromatic product and used in our daily lives.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgmentsThis work was financially supported by the National High Technology Research and Development Program (No. 2016YFA0200303), the Beijing Natural Science Foundation (Nos. L172046, 2192057), the National Natural Science Foundation of China (Nos. 31771095, 21875254 and 51573188).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.01.041.
| [1] |
M. Taverna, A.E. Baillet, D. Baylocq, J. Assoc. Off. Ana. Chem. 73 (1990) 206-210. |
| [2] |
I. Oey, M. Lille, A. Van Loey, M. Hendrickx, Trends Food. Sci. Tech. 19 (2008) 320-328. DOI:10.1016/j.tifs.2008.04.001 |
| [3] |
M.B. Pashazanousi, M. Raeesi, S. Shirali, Asian J. Chem. 24 (2012) 4331-4334. |
| [4] |
C. Dobetsberger, G. Buchbauer, Flavour Frag. J. 26 (2011) 300-316. DOI:10.1002/ffj.2045 |
| [5] |
D.L. Berthier, I. Schmidt, W. Fieber, et al., Langmuir 26 (2010) 7953-7961. DOI:10.1021/la904832d |
| [6] |
S.J. Gilpin, X.Y. Hui, H.I. Maibach, Dermatitis 20 (2009) 200-207. DOI:10.2310/6620.2009.08117 |
| [7] |
S. Chakraborty, J. Carbohyd. Chem. 36 (2017) 1-19. DOI:10.1080/07328303.2017.1347668 |
| [8] |
D. Dreau, L.J. Moore, M.P. Alvarez-Berrios, et al., J. Biomed. Nanotechnol. 12 (2016) 2172-2184. DOI:10.1166/jbn.2016.2318 |
| [9] |
Y.Y. Huang, D. Weng, L. Han, et al., Nanosci. Nanotechnol. Lett. 9 (2017) 1639-1648. DOI:10.1166/nnl.2017.2534 |
| [10] |
A.L. Lin, S.Z. Li, C.H. Xu, et al., Biomater. Sci-UK 7 (2019) 211-219. DOI:10.1039/C8BM00386F |
| [11] |
J. Rautio, N.A. Meanwell, L. Di, M.J. Hageman, Nat. Rev. Drug Discov. 17 (2018) 559-587. DOI:10.1038/nrd.2018.46 |
| [12] |
X. Guo, L. Wang, K. Duval, et al., Adv. Mater. 30 (2018) 1705436. DOI:10.1002/adma.201705436 |
| [13] |
S.J. Sonawane, R.S. Kalhapure, T. Govender, Eur. J. Pharm. Sci. 99 (2017) 45-65. DOI:10.1016/j.ejps.2016.12.011 |
| [14] |
Q.N. Lin, C.Y. Bao, Y.L. Yang, et al., Adv. Mater. 25 (2013) 1981-1986. DOI:10.1002/adma.201204455 |
| [15] |
H.Y. Yoon, S.S. Kwak, M.H. Jang, et al., Int. J. Pharmaceut. 523 (2017) 229-237. DOI:10.1016/j.ijpharm.2017.03.045 |
| [16] |
Y. Shtenberg, M. Goldfeder, H. Prinz, et al., Int. J. Biol. Macromol. 111 (2018) 62-69. DOI:10.1016/j.ijbiomac.2017.12.137 |
| [17] |
A. Mandal, R. Bisht, I.D. Rupenthal, A.K. Mitra, J. Control.Release 248 (2017) 96-116. DOI:10.1016/j.jconrel.2017.01.012 |
| [18] |
P.E. Saw, M.Y. Yu, M. Choi, et al., Biomaterials 123 (2017) 118-126. DOI:10.1016/j.biomaterials.2017.01.040 |
 2021, Vol. 32
2021, Vol. 32 

