b College of Biological, Chemical Sciences and Engineering, Jiaxing University, Jiaxing 314001, China;
c Department of Chemistry and Biochemistry, The University of Texas at Austin, Austin 78712, United States
Construction of heterometallic nanoclusters with d-block metals and lanthanide(Ⅲ) ions has recently gained considerable attention due to their unique optical, electrical and magnetic properties [1, 2]. Fluorescent response towards analytes (i.e., cations, anions and small molecules) are currently of interest because of its potential application in environment, biology and medicine [3-9]. For example, the development of fluorescent molecular sensors for the recognition of alkali metal ions such as lithium (Li+), sodium (Na+) and potassium (K+) is critical for the biomedical diagnosis [10-12]. Luminescent lanthanide complexes display unrivalled spectroscopic properties, including large Stokes shifts, sharp emission bands and long emission lifetimes. Lanthanide complexes with Nd(Ⅲ), Yb(Ⅲ) and Er(Ⅲ) ions may show near-infrared (NIR) luminescence, which can penetrate deep into the tissues because biological systems are almost transparent in the wavelength range from 900 nm to 1600 nm. So far, many visible luminescent Eu(Ⅲ)- and Tb(Ⅲ)-based complexes have been used as sensors for the detection ofmetal ions [13-18]. However, there are veryfew reports on the NIR luminescent lanthanide-based complexes used as metal ion probes, especially to alkali metal ions.
Metal chromophores with d-block transition-metal ions such as Zn(Ⅱ), Cd(Ⅱ), Pt(Ⅱ) and Ru(Ⅱ) may act as efficient sensitizers for the NIR lanthanide luminescence. Compartmental Schiff bases with two dissimilar metal-binding sites have been widely used to synthesize 3d-4f heteronuclear complexes [19, 20]. As part of our current research on the luminescent response of d-4f complexes towards various analytes [21-23], we report here one 6-metal Zn-Nd complex [Zn2Nd4L2(OAc)10(OH)2(CH3OH)2] (1) with Schiff base ligand bis(3-methoxysalicylidene)ethylene-1,2-phenylenediamine (H2L, Fig. 1). 1 exhibits nanoscale rectangular structure (8 × 11 × 28 Å). Interestingly, 1 shows luminescent response towards metal ions, especially to Li+, Na+ and K+ ions.
|
Download:
|
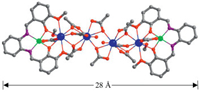
| Fig. 1. A view of the crystal structure of 1 (Nd3+: blue; Zn2+: green). | |
The Schiff-base ligand H2L was prepared according to the literature report [24]. Reaction of H2L with Zn(OAc)2·2H2O and NdCl3·6H2O in refluxing MeOH gave the Zn-Nd complex 1. The yellow crystalline product of 1 was obtained by the slow diffusion of diethyl ether into the reaction solution, and zinc chloride ([ZnCl2(H2O)CH3OH] was found in the crystalline solid of the product CCDC No. 1971956. As shown in Fig. 1, the X-ray structure of 1 reveals a centrosymmetric core with 2 equiv. ZnLn2L moieties, which are linked together by two OAc- anions. In the ZnLn2L moiety, Zn2+ ion is bound in the O2N2 cavity of the Schiff base ligand, and shows a square pyramidal geometry. For the two Nd3+ ions, one is bound by the O2O2 set of the Schiff base ligand and another one is coordinated with five OAc- and one OH- anions. The Zn2+ ion is linked with the former Nd3+ ion by the Schiff base ligand and two OAc- anions, with a separation of 3.402 Å. Both Nd3+ ions are nine-coordinated and exhibit a single cap square antiprism geometry. In 1, all Nd3+ ions are linked together by ten OAc- anions and the average Nd(Ⅲ)···Nd(Ⅲ) distance is 4.088 Å. The OAc- anions exhibit μ2 and μ3 coordination modes with the Nd3+ ions. The Schiff base ligand is bound to metal ions through its N and O atoms. In 1, there are two methanol molecules coordinated with the Nd(Ⅲ) ions. The metric dimensions of 1 measure approximately 8 × 11 × 28 Å. The Zn—N, Zn—O and Nd—O bond lengths range from 2.036 Å–2.047 Å, 1.994 Å–2.012 Å, and 2.314 Å– 2.679 Å, respectively.
The panoramic scanning electron microscopy (SEM) image and energy dispersive X-ray spectroscopy (EDX) spectrum of 1 are shown in Fig. 2. The molar ratio of Zn/Nd in the EDX spectrum of 1 is found to be about 1:1, consistent with its crystal structure. Thermogravimetric analysis exhibits that 1 loses about 5% of the weight when it is heated at 100 ℃ (Fig. S2 in Supporting information), due to the escaping of solvent molecules in the product. It is found that, when the crystalline product of 1 is separated from its mother solution, it rapidly loses crystallinity. The powder XRD pattern of 1 shows many weak diffraction peaks with large background peaks (Fig. S3 in Supporting information), confirming its amorphous nature. The Zn-Nd complex starts to discompose when it is heated at 200 ℃ (Fig. S2).

|
Download:
|
| Fig. 2. (a) Scanning electron microscopy image and (b) energy dispersive X-ray spectroscopy spectrum of 1. | |
The luminescence properties of 1 was studied in CH3CN and the solid state. As shown in Fig. S4 (Supporting information), compared with the absorption bands of the free H2L, those of 1 are red-shifted. Excited by ligand-centered absorption bands, 1 shows typical NIR luminescence of Nd3+ (4F3/2→4Ij/2 transitions, j = 9, 11 and 13) (Fig. S5 in Supporting information). For the lanthanide luminescence of 1, the most intense line is at 1054 nm (4F3/2→4I11/2). The complex shows similar luminescence spectra in the solution and the solid state.
For the lanthanide luminescence, 1 exhibits broad excitation bands, in agreement with their absorption spectra. This confirms that the energy transfer from Zn/L centers to Nd3+ ions occurs in 1. The Nd3+ ion has many excitation energy levels lying above the emissive 4F3/2 state at 11,300 cm-1 [25], which helps to accept energy from the Zn/L center. The NIR emission lifetime (τ) of 1 in CH3CN is 6.2 ms (Fig. S5). Thus, the intrinsic quantum yield (ΦLn) of Nd3+ in 1 is calculated to be 2.5% (ΦLn = τ/τ0, τ0 = 250 μs, the natural lifetime of Nd3+ ion) [26]. The overall NIR emission quantum yield (Φem) of 1 is 0.8%. The energy transfer efficiency (ηsens = Φem/ΦLn) from Zn/L centers to lanthanide ions in 1 is estimated to be 32% [27]. Solvent molecules such as H2O can efficiently quench the lanthanide luminescence through nonradiative energy exchange from Ln3+ to the solvent molecules [28]. The overall NIR emission quantum yield of 1 in DMF/H2O (1:1) is found to be 0.5%, which is lower than that in CH3CN.
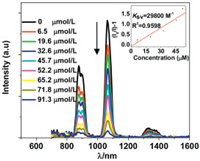
There are total 30 electronegative O atoms from Schiff base ligands and anions (OAc- and OH-) in the structure of 1, which may help to form intermolecular electrostatic force with added cations. The lanthanide luminescent response of 1 towards metal ions Li+, Na+, K+, Co2+, Cd2+, Ag+, Cr3+, Ni2+, Al3+, Mn2+, Zn2+ and Mg2+ (metal acetates) have been investigated in DMF. Interestingly, the addition of all metal ions decreases the lanthanide luminescence of 1 (Fig. 3 and Fig. S6 in Supporting information). It is noticeable that, the addition of alkali metal ions Li+, Na+ and K+ causes a more rapid decrease of the luminescence intensity than the addition of other metal ions. The emission intensities at 1054 nm of 1 are decreased more than 50% when the concentrations of added Li+, Na+ and K+ ions are 45.7 μmol/L, 47.4 μmol/L and 61.4 μmol/L, respectively. These concentrations are much lower than those of other ions (Fig. S6). For example, the concentrations of added Zn2+ and Mg2+ ions to reduce the emission intensities of 1 by half are 1762 μmol/L and 1958 μmol/L, respectively. These results indicate that, compared with other metal ions, 1 shows high sensitivity to Li+, Na+ and K+ ions through lanthanide luminescent response.
|
Download:
|
| Fig. 3. Lanthanide luminescent response of 1 (50 μmol/L) to the addition of different concentrations of Li+ ion in DMF. λex =395 nm. | |
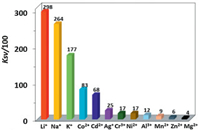
The Stern-Volmer equation KSV = (I0/I - 1)/[A] can be used to calculate the emission quenching efficiencies (KSV) of 1 to metal ions [29], where [A] is the concentration of added metal ion, and I0 (or I) is the emission intensity of 1 at 1054 nm before (or after) the addition of metal ion. The KSV values of 1 towards Li+, Na+ and K+ ions are found to be 2.98 × 104 L/mol, 2.64 × 104 L/mol and 1.77 × 104 L/mol, respectively. These values are much greater than those to other metal ions (Fig. 4). For example, the KSV values of 1 towards Zn2+ and Mg2+ ions are 0.06 × 104 L/mol and 0.04 × 104 L/mol, respectively. The limits of detection (LOD) of 1 to metal ions can be estimated by 3σ/KSV [30], where s is the standard deviation. The LOD data of 1 to Li+, Na+ and K+ ions are calculated to be 0.47 μmol/L, 0.53 μmol/L and 0.79 μmol/L, respectively, which are comparable to those reported in the literature [31-35]. These results indicate that 1 shows NIR lanthanide luminescent response to these alkali metal ions at ppm level. 1 has similar KSV and LOD data to Li+, Na+ and K+ ions, thus it needs to use other elemental analysis methods to furtherly differentiate them in practical application.
|
Download:
|
| Fig. 4. Lanthanide luminescence quenching efficiencies (KSV) of 1 (50 μmol/L) towards metal cations. | |
The added metal ions may affect the ligand-to-metal energy transfer process in 1 due to their perturbation to the electronic structure and excited state of the ligand, resulting in so-called chelation enhancement of the quenching emission (CHEQ) effect [36]. For example, with unfilled d electronic configurations, 3d-block metal ions Co2+, Cr3+, Ni2+ and Mn2+ have d-d transitions, which may consume the excitation energy of lanthanide ions through f→d energy transfers and decrease the lanthanide luminescence of 1 [37]. Zn2+ and Mg2+ ions have d10 and d0 electronic configurations, respectively, which prevent the quenching of lanthanide luminescence through d-d transitions, and 1 has low KSV values to these two metal ions. The interactions between the added metal ions and 1 play a key role in the lanthanide luminescent response. 1 shows higher KSV values to alkali metal ions than to other metal ions, probably due to the formation of stronger intermolecular interactions between the alkali metal ions and 1. For example, alkali metal ions may interact with macrocyclic compounds and framework complexes through cation-π interactions due to their suitable ionic size [38-41]. The interactions between the added alkali metal ions and 1 were confirmed by UV– vis spectra [42]. As shown in Fig. S7 (Supporting information), the red-shift of the absorption bands of 1 with the addition of alkali metal ions indicates the formation of interactions between the metal ions and 1. The CHEQ effect of alkali metal ions to 1 was investigated by the measurement of emission lifetimes and quantum yields after the addition of metal ions. For example, when the concentration of the added Li+ ion is 45.7 μmol/L, the luminescence lifetime (τ) and the overall emission quantum yield (Φem) of 1 are 5.6 ms and 0.3%, respectively. Thus, the intrinsic quantum yield (ΦLn) is calculated to be 2.2%, and the efficiency (ηsens) of the energy transfer is estimated to be 13.6%, which is lower than that without the Li+ ion. These results indicate that the addition of alkali metal ions may decrease the efficiency of the energy transfer process in 1.
In summary, a nano-rectangular Zn-Nd complex 1 with molecular size of 10 × 14 × 18 Å was successfully synthesized using a Schiff base ligand, and identified with X-ray crystallography. Upon the excitation of absorption bands, 1 exhibits the typical NIR luminescence of Nd3+ ion. The addition of Li+, Na+, K+, Co2+, Cd2+, Ag+, Cr3+, Ni2+, Al3+, Mn2+, Zn2+ and Mg2+ ions decreases the lanthanide luminescence of 1. It shows high sensitivity to alkali metal ions (Li+, Na+ and K+) at ppm level. Further studies focused on the luminescent response of d–f complexes to other analytes are in progress.
Conflicts of competing interestThe authors declare that there are no conflicts of interest.
AcknowledgmentThe work was supported by the National Natural Science Foundation of China (No. 21771141).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.03.016.
| [1] |
B. Wang, Z. Zang, H. Wang, et al., Angew. Chem. Int. Ed. 52 (2013) 3756-3759. DOI:10.1002/anie.201210172 |
| [2] |
J.B. Peng, Q.C. Zhang, X.J. Kong, et al., J. Am. Chem. Soc. 134 (2012) 3314-3317. DOI:10.1021/ja209752z |
| [3] |
X. Qi, Y. Jin, N. Li, et al., Chem. Commun. 53 (2017) 10318-10321. DOI:10.1039/C7CC05345B |
| [4] |
X. Sun, Y. Wang, Y. Lei, Chem. Soc. Rev. 44 (2015) 8019-8061. DOI:10.1039/C5CS00496A |
| [5] |
J. Jankolovits, C.M. Andolina, J.W. Kampf, K.N. Raymond, V.L. Pecoraro, Angew. Chem. Int. Ed. 50 (2011) 9660-9664. DOI:10.1002/anie.201103851 |
| [6] |
Q. Wen, Y. Tang, K. Li, Y. Zheng, Soft Mater. 17 (2019) 350-358. DOI:10.1080/1539445X.2019.1606013 |
| [7] |
X. Yang, Y. Zhang, W. Liu, J. Gao, Y. Zheng, Polyhedron 171 (2019) 389-395. DOI:10.1016/j.poly.2019.07.040 |
| [8] |
L. Zhou, J.F. Liao, Z.G. Huang, et al., Angew. Chem. Int. Ed. 58 (2019) 5277-5281. DOI:10.1002/anie.201814564 |
| [9] |
L. Zhou, J.F. Liao, Z.G. Huang, et al., Angew. Chem. Int. Ed. 58 (2019) 15435-15440. DOI:10.1002/anie.201907503 |
| [10] |
H. Kager, W.J. Wadman, G.G. Somjen, Neurophysiology 84 (2000) 495-512. DOI:10.1152/jn.2000.84.1.495 |
| [11] |
V. Petrilli, S. Papin, C. Dostert, et al., Cell Death Differ. 14 (2007) 1583-1589. DOI:10.1038/sj.cdd.4402195 |
| [12] |
J.K. Tusa, H. He, J. Mater. Chem. 15 (2005) 2640-2647. DOI:10.1039/b503172a |
| [13] |
B. Chen, L. Wang, Y. Xiao, et al., Angew. Chem. Int. Ed. 48 (2009) 500-503. DOI:10.1002/anie.200805101 |
| [14] |
P.F. Shi, H.C. Hu, Z.Y. Zhang, G. Xiong, B. Zhao, Chem. Commun. 51 (2015) 3985-3988. DOI:10.1039/C4CC09081K |
| [15] |
Y. Qiu, H. Deng, J. Mou, et al., Chem. Commun. (2009) 5415-5417. |
| [16] |
G.L. Liu, Y.J. Qin, L. Jing, G.Y. Wei, H. Li, Chem. Commun. 49 (2013) 1699-1701. DOI:10.1039/C2CC37140E |
| [17] |
Z. Guo, H. Xu, S. Su, et al., Chem. Commun. 47 (2011) 5551-5553. DOI:10.1039/C1CC10897B |
| [18] |
A. Thibon, V.C. Pierre, J. Am. Chem. Soc. 131 (2009) 434-435. DOI:10.1021/ja8077889 |
| [19] |
M. Sakamoto, K. Manseki, H. Okawa, Coord. Chem. Rev. 219 (2001) 379-414. |
| [20] |
R.E.P. Winpenny, Chem. Soc. Rev. 27 (1998) 447-452. DOI:10.1039/a827447z |
| [21] |
C. Wang, X. Yang, S. Wang, et al., J. Mater. Chem. C 6 (2018) 865-874. DOI:10.1039/C7TC04101B |
| [22] |
D. Jiang, X. Yang, X. Zheng, et al., J. Mater. Chem. C 6 (2018) 8513-8521. DOI:10.1039/C8TC02426J |
| [23] |
J. Liu, X. Yang, T. Zhu, et al., RSC Adv. 9 (2019) 40017-40022. DOI:10.1039/C9RA09409A |
| [24] |
F. Lam, J.X. Xu, K.S. Chan, J. Org. Chem. 61 (1996) 8414-8418. DOI:10.1021/jo961020f |
| [25] |
N.M. Shavaleev, G. Accorsi, D. Virgili, et al., Inorg. Chem. 44 (2005) 61-72. DOI:10.1021/ic048875s |
| [26] |
S.I. Klink, L. Grave, D.N. Reinhoudt, et al., J. Phys. Chem. A 104 (2000) 5457-5468. DOI:10.1021/jp994286+ |
| [27] |
J.C.G. Bünzli, C. Piguet, Chem. Soc. Rev. 34 (2005) 1048-1077. DOI:10.1039/b406082m |
| [28] |
S. Yanagida, Y. Hasegawa, K. Murakoshi, et al., Coord. Chem. Rev. 171 (1998) 461-480. DOI:10.1016/S0010-8545(98)90069-8 |
| [29] |
Y. Xiao, Y. Cui, Q. Zheng, et al., Chem. Commun. 46 (2010) 5503-5505. DOI:10.1039/c0cc00148a |
| [30] |
X. Qi, Y. Jin, N. Li, et al., Chem. Commun. 53 (2017) 10318-10321. DOI:10.1039/C7CC05345B |
| [31] |
B. Sui, X. Yue, M.G. Tichy, T. Liu, K.D. Belfield, Eur. J. Org. Chem. (2015) 1189-1192. |
| [32] |
B. Sui, X. Yue, B. Kim, K.D. Belfield, ACS Appl. Mater. Interfaces 7 (2015) 17565-17568. DOI:10.1021/acsami.5b04506 |
| [33] |
G. Ji, J. Liu, X. Gao, et al., J. Mater. Chem. 5 (2017) 10200-10205. DOI:10.1039/C7TA02439H |
| [34] |
Y. Fu, X.J. Jiang, Y.Y. Zhu, et al., Dalton Trans. 43 (2014) 12624-12632. DOI:10.1039/C4DT01453G |
| [35] |
Q. Meng, H. Jia, P. Succar, et al., Biosens. Bioelectron. 74 (2015) 461-468. DOI:10.1016/j.bios.2015.06.077 |
| [36] |
Treto-Suárez M.A., Y. Hidalgo-Rosa, E. Schott, X. Zarate, D. Páez-Hernández, J. Phys. Chem. A 123 (2019) 6970-6977. DOI:10.1021/acs.jpca.9b03366 |
| [37] |
X. Wen, Y. Zhao, L. Wang, M. Zhang, D. Gao, J. Rare Earths 25 (2007) 679-683. DOI:10.1016/S1002-0721(08)60006-X |
| [38] |
Z. Han, Z. Xiao, M. Hao, et al., Cryst. Growth Des. 15 (2015) 531-533. DOI:10.1021/cg501259g |
| [39] |
X. Zhou, F. Su, Y. Tian, et al., J. Am. Chem. Soc. 133 (2011) 18530-18533. DOI:10.1021/ja207345s |
| [40] |
B.J. Müller, S.M. Borisov, I. Klimant, Adv. Funct. Mater. 26 (2016) 7697-7707. DOI:10.1002/adfm.201603822 |
| [41] |
B. Sui, X. Yue, B. Kim, K.D. Belfield, ACS Appl. Interfaces 7 (2015) 17565-17568. DOI:10.1021/acsami.5b04506 |
| [42] |
C.L. Liu, L.P. Zhou, D. Tripathy, Q.F. Sun, Chem. Commun. 53 (2017) 2459-2462. DOI:10.1039/C7CC00189D |
 2021, Vol. 32
2021, Vol. 32 

