b Key Laboratory of Bioelectrochemistry & Environmental Analysis of Gansu Province, College of Chemistry & Chemical Engineering, Northwest Normal University, Lanzhou 730070, China
Colorimetric sensing strategies have attracted considerable attention due to their unique advantages such as simple, low-cost, easy-to-use, affordable, and good practicality [1-5]. Different from highly accurate diagnostic related analyses and tests assisted by complex instruments, these methods exhibit huge application prospects in practical applications and resource-limited regions. Recognition of target analytes in different sensing events usually can be finished relying on the sensitive color change as the sensing readout, and which can be simply read out by the naked eye without the assistance of expensive or sophisticated instruments. It therefore is more suitable for field analysis and point-of-care testing (POCT) [6-12].
More recently, the introduction of nanozymes further accelerate this attracting colorimetric field and exhibit more promising application prospects. As an ideal candidate for artificial mimic enzymes, nanozymes specifically refer to the nanomaterials with enzyme-mimetic catalytic activity, which can simulate the catalytical function of natural enzymes well in some important biochemical and chemical reactions [13-15]. Since the first finding of intrinsic peroxidase-like activity (POX) of Fe3O4 nanoparticles in 2007 [16], diversified nanozymes with intrinsic enzyme-like catalytic activities were extensively explored [17, 18], such as metal nanoparticles [19-22], carbon nanomaterials [23], metal oxides [24-26] and so on [27, 28]. Compared to natural enzymes, these developed nanozymes have higher stability, lower cost and simple preparation [14, 15, 29]. On the other hand, nanozymes can provide activity-dependent colorimetric sensing response by using a number of organic indicators as the peroxidase or oxidase substrates, such as 3, 3′, 5, 5′-tetramethylbenzydine (TMB), o-phenylenediamine (OPD) and 2, 2-azinobis-(3-ethylben-zothizoline-6-sulfonic acid (ABTS). All these unique merits further enable nanozymes more potential in colorimetric sensing applications, as they can provide a series of simple and inexpensive devices and tools that armed with simple signal color readout [30-34].
Nanozymes-based strategies have been recently demonstrated that can effectively accelerate and advance the development of colorimetric sensors for chem/biosensing events. However, the majority of existing nanozymes-based colorimetric assays are the limited specificities and catalytic activities of nanozymes. It therefore is highly desirable to develop effective strategies to improve the performance and advance the practical application for nanozymes-based colorimetric sensors [35]. Many efforts have demonstrated that several factors are closely related to the catalytic activity of nanozymes, such as pH and temperature [16, 36, 37], size [21, 38], morphology [39, 40] and composition [41, 42]. Based on these findings, lots of strategies were recently explored to tune the catalytic activity of nanozymes by focusing on the above factors, which greatly accelerating nanozymes-based colorimetric sensing applications such as quantitative detection of disease-specific biomarkers [43-45], food safety monitoring [46-48] and environmental pollution detection [49, 50].
The key challenge in the design of these nanozymes activity controllable systems and the corresponding colorimetric sensors is selectively introducing the target analytes into the nanozymes system. Based on this, the detection events can be simply achieved by using the sensitive bare-eye-detectable color readout as shown in Fig. 1 [9, 51]. To this end, a new route based on aggregation-induced nanozymes was explored to effectively regulate nanozyme activity, and greatly accelerate the development of nanozymes-based colorimetric sensors. To date, more and more evidences have been made that the catalytic activity of nanozymes could be modulated and altered upon the aggregation effect of nanozymes. More significantly, these methods have demonstrated good performance for constructing activity-controllable nanozymes and better their colorimetric sensing applications. We herein present this work to exclusively review the recent progress in the development of aggregation-induced nanozymes based colorimetric sensors along with the discussions on the challenges and perspectives of this rapidly advancing direction. We want to provide critical guidance with this review for aggregation-induced nanozymes based colorimetric sensors.
|
Download:
|
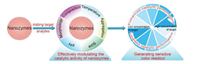
| Fig. 1. Schematic representation of target-modulated nanozymes activity and their corresponding colorimetric system. | |
Many efforts have demonstrated that aggregation-induced nanoparticles systems usually can exhibit excellent optical properties [52-57], such as the sensitive surface plasmon resonance (SPR) that extensively used for fabricating noble metal nanoparticles based colorimetric assays as the plasmon-enhanced optical detection tools [58, 59]. Those smart color readout based systems perform well based on the analyte-induced formation of aggregates of noble metal nanoparticles as displayed in Fig. 2A, which maybe attributed to the changed morphology and size as well as the resultant external surface area and active sites affected by aggregation-induced effect [60-62]. It subsequently was demonstrated that such aggregation-induced properties associated with the nanoparticle morphology and size are more useful for fabricating the corresponding colorimetric sensing tools for different target analytes.

|
Download:
|
| Fig. 2. Schematic representation of colorimetric sensors based on (A) surface plasmon resonance of noble metal nanoparticles, (B) aggregation-induced emission of nanoclusters and (C) aggregation-induced controllable activities of nanozymes. | |
Another good example is aggregation-induced emission (AIE) property was developed by Tang on the basis of the fact that aggregation-induced light emission for silole molecules [63]. The enhanced emission performance in the aggregation-induced fluorescent system has been rapidly extended from organic molecules to polymeric systems and nanomaterials, and resulted in an extensive and successful fabrication of AIE-based colorimetric sensors [64, 65]. In such sensing system, the aggregates both of organic molecules and polymeric substances, as well as nanostructures lead to the obvious change on their corresponding size or morphology, which further results in the optical properties variation and finally can be used in fluorometric sensing application as the sensing readout for different target analytes (Fig. 2B). All these aggregation-induced observations from SPR-based colorimetric and AIE-based fluorometric strategies both demonstrated that the dispersion and aggregation status of most noble metal nanoparticles or nanostructures can promote the generation of elegant color or fluorescent readout, and further accelerating the advances and development of the corresponding colorimetric and fluorometric recognition and sensing events [66-69]. These SPR-based colorimetric and AIE-based fluorometric strategies as a direct signal generation method were extensively used in the fabrication of the sensitive assays. Such aggregation-induced effect also inspired more researchers to expand other types of colorimetric sensors such as build the sensing platforms with an indirectly sensing signal generation mode by using nanozymes-catalyzed process [70-73].
Taking the advantages of SPR effect of noble metal nanostructures [74] and AIE properties of organic molecules [75, 76] and polymeric systems [77] as well as nanomaterials [78-80], the aggregation-induced effect was also recommended to first modulate nanozymes activity and then fabricate the corresponding color readout based colorimetric sensors. As shown in Fig. 2C, the morphology and size of nanozymes may be significantly altered through the aggregation-induced procedure, which further leads to a successful regulation on the surface area and active site blockages of nanozymes. All these aggregation-induced changes on the nanozymes inherent properties further lead to the effective regulation for nanozymes catalytic activity. By linking the resultant nanozymes with aggregation-induced controllable activity with some nanozymes catalyzed chromogenic substrates such as TMB, OPD and ABTS, efforts focused on the fabrication of nanozymes-based colorimetric assays have been successfully devoted.
To confirm and verify the above principle and performance, the modulation of nanozymes activity mediated by nanozymes aggregates were investigated in the early stages of this attractive research direction. As a good example, DNA was widely proposed as the sensitive inducer and modulator to investigate the corresponding aggregation of nanozymes. As well known, DNA as an important biomolecule has exhibited a powerful modulating ability for controlling the peroxidase-like activities of various nanozymes. For example, the peroxidase-like activity of two-dimensional (2D) metal-organic framework (MOF) nanosheets-based nanozymes were demonstrated that could be flexibly modulated by single-stranded DNA (ssDNA), and exhibited a significant enhancement for TMB oxidation owing to the good adsorption of ssDNA on the nanozyme surface [81]. Nanoparticles as the sensing substrate usually exhibit a high adsorbing ability to some DNA molecules due to their high specific surface area [82]. The phosphate backbone of DNA and the phosphate group of lipids both can accelerate the interaction performance between nanoparticles surface and DNA [83, 84]. Based on this fact, Zhao and Liu et al. investigated the promotion and inhibition effect of the oxidase-mimicking activity of nanoceria in the presence of DNA [85]. More importantly, it is worth noting that DNA exhibits the sensitive modulating effect for nanoceria activity but strongly depending on its concentration, as well as the types of buffer and substrate as shown in Fig. 3A. It is also observed that the catalytic activity of nanoceria nanozyme toward TMB oxidation was greatly enhanced by the low DNA content in the phosphate buffer. All these results indicate that the effective DNA-induced aggregation of nanoceria nanozymes can be obtained upon the optimal conditions, and thus providing a guideline for developing nanozymes based colorimetric detection tools for DNA in biological samples [70-73].

|
Download:
|
| Fig. 3. Schematic illustration of DNA-induced aggregation of (A) CeO2 nanozyme in different buffer systems and the resultant enhancement and inhibition performance on CeO2 activity. Copied with permission [85]. Copyright 2020, Wiley. (B) Fe3O4NP@pSiO2 nanozyme and its activity inhibition for colorimetric hgDNA sensing. Copied with permission [86]. Copyright 2019, Elsevier B.V. | |
On the other hand, Öğüt et al. recently found that DNA can significantly and particularly induce the extensive and irreversible aggregation of nanozymes [86]. Such long-chain biomolecules of DNA-based clumping effect for nanozymes particles in turn greatly suppressed the catalytic activity of nanozymes, and preventing the corresponding colorimetric sensing application of nanozymes (Fig. 3B). By investigating in detail, they found that DNA-induced nanozyme aggregates exhibited very limited surface area and lots of active site blockages due to the adsorption of biomolecule chains of DNA. All these are largely responsible for inhibiting the peroxidase-mimetic activity of nanozyme.
Both above two good examples strongly indicate that aggregates of nanozymes through analytes-mediated design can further trigger a specific activity regulation for nanozymes, attributing to the altered morphology and size of nanozymes by aggregation-induced effect, especially due to the accordingly changed surface area and lots of active site blockages of nanozymes. All these aggregation dependent properties are likely to be largely responsible for modulating the catalytic activity of nanozymes, which can further provide a potential route for advancing the development of nanozymes-based colorimetric detection tools.
3. Classification of aggregation-induced nanozymes based colorimetric sensorsTo date, more and more new thoughts focusing on the color readout generation were explored to stimulate and advance nanozymes based colorimetric detection tools. Typically, the color readout in an aggregation-induced nanozymes based colorimetric sensor is built relying on nanozymes activity modulation and their corresponding catalyzed chromogenic reaction, which further leads to a successful signal generation and target recognition. Therefore, it is clear that the flexible modulating effect of aggregation-induced nanozymes for the catalytic activity of nanozymes provides a possible chance to fabricate colorimetric analysis and testing tools in different chem/biosensing fields. As far as the target-induced or analyte-induced nanozymes aggregates, diversified substances in various fields such as disease diagnostics and environmental monitoring have been developed as the new technique not only for observing the aggregation-induced nanozymes effect but also for constructing the corresponding colorimetric detection tools.
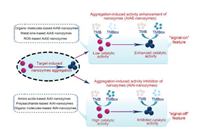
According to the modulating performance of nanozymes activity by the aggregation-induced process, such systems can be divided into the following two types, as shown in Fig. 4. One is aggregation-induced activity enhancement of nanozymes (AIAE-nanozymes) mediated by different target analytes such as metal ions [87], small organic molecules [88] and reactive oxygen species (ROS) species in the biological system [89]. Through this mode, the proposed nanozymes maybe form aggregates in the liquid phase solution and exhibit an enhanced mimic catalytic activity, which can further produce the sensitive sensing unit for target recognition by generating the color change via nanozymed catalyzed chromogenic reaction. It is worth to note that the color signal in this mode can be classified as a "signal-on" model, because of the enhanced color change readout from catalyzed chromogenic reaction of the peroxidase substrate (such as TMB, OPD and ABTS) by the aggregation-induced activity enhancement of nanozymes. In addition, it also provides the possible solution for simply infusing the colorimetric method into another field such as fluorometric technology by flexibly converting color readout into the fluorescent signal [90-93]. By following this concept, AIAE-nanozymes have demonstrated more potential in the development of multimodal sensing tools for different analysis field.
|
Download:
|
| Fig. 4. Two types of nanozymes-based colorimetric assays based on the aggregation-induced activity enhancement and inhibition of nanozymes. | |
On the other hand, the formation of nanozyme aggregation in the solution system sometimes may also exhibit an inhibited enzyme mimic activity [94, 95]. Based on this feature, aggregation-induced activity inhibition of nanozymes (AIAI-nanozymes) as a new modulating strategy for nanozymes activity was recently explored. The emergence of such AIAI-nanozymes not only enriches the methodology of controllable activity for nanozymes, but also endows another nanozymes-based colorimetric detection tool with an obvious sensing performance in a "signal-off" model. In such systems, the sensing units usually are designed relying on the inhibited activity and the resultantly decreased nanozymes catalyzed chromogenic reaction, which further leads to a suppressed generation of the color response associated with the target content and the corresponding analyte-induced nanozymes aggregation. Some target analytes have been demonstrated to possess such a unique regulation features for the different nanozymes, such as metal ions, polysaccharide molecules [96], organic molecules [97] and amino acids [98].
As a steadily growing interest in nanozymes-based colorimetric field, this newly-developing aggregation-induced nanozymes based colorimetric sensors with "signal-on" and "signal-off" features greatly improve the analytical performance, and significantly opening the door for fabricating different nanozymes based sensors and advancing the development in this attractive direction. It also provides a route to evolve the concept of nanozymes based colorimetric analysis and testing methods by using the aggregation-induced modulation of nanozymes activity. More significantly, it is worth mentioning that the sensing signal with the turn-off feature in AIAI-nanozymes based sensors usually is quenched by analyte directly, therefore exhibiting some shortcomings such as high false-positive results caused by the other quenchers and environmental stimulus, in which the target-induced color signal often may be affected by environmental factors [99, 100]. However, AIAE-based sensors with turn-on feature signals often exhibit lower detection background than "turn off" detection mode.
4. Nanozymes-based colorimetric sensors based on aggregation-induced activity enhancementAmong the superior features of AIAE-nanozymes, the most prominent is their good activated activity performance for nanozymes mediated by the aggregation-induced effect. It can effectively promote the sensing signal accumulation due to the enhanced nanozymes activity toward chromogenic reaction with significantly increased catalytic capability. It therefore greatly accelerate the development of nanozymes-based colorimetric sensors. To date, metal ions and small organic molecules have been explored and applied into the fabrication of AIAE-nanozymes based colorimetric assays.
As a simple and direct method for regulating the peroxidase-like activity of nanozymes, metal ions induced aggregation demonstrated more benefits in AIAE-nanozymes based colorimetric sensing applications. Generally, metal ions induced aggregates of nanomaterials include two key elements, the capping agent of nanoparticles or other nanostructures surface, and the unique binding affinity between the metal ions and the capping element. Very similar to the metal ions induced SPR phenomena, some metal nanoparticles with enzyme mimic activities also exhibited a common metal ions induced aggregation effect. More significantly, through the formation of aggregates, the intrinsic catalytic activity of nanozymes usually can be greatly enhanced and promoted. The resultant nanozymes with high catalytic capability then provide more benefits for broadening the application of nanozymes-based colorimetric detection tools.
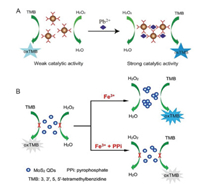
As well known, noble metal nanoclusters like Au nanoclusters (AuNCs) usually have better catalytic activity and have been extensively applied in chem/biosensing applications. However, AuNCs system only shows lower activity toward the peroxidase substrate of TMB as a nanoenzyme. Liao and co-worker found that Pb2+ could induce an obvious AuNCs aggregation effect [87], which subsequently leads to an interesting aggregation-induced catalytic activity enhancement for AuNCs. Based on this finding, a reliable and simple colorimetric assay for Pb2+ detection relying on AIAE-nanozymes was successfully developed, as shown in Fig. 5A. The developed modulation strategy for the controllable morphology of AuNCs not only facilitated the deep understanding of AuNCs aggregation, but also provided a new insight for extending the nanoparticles-based nanozymes with aggregation-induced properties in the applications of colorimetric sensors.
|
Download:
|
| Fig. 5. Schematic illustration of (A) Pb2+-induced aggregation based activity enhancement of AuNCs nanozyme for colorimetric Pb2+ detection. Copied with permission [87]. Copyright 2017, Royal Society of Chemistry. Schematic illustration of (B) Fe3+-induced aggregation based activity enhancement of MoS2 QDs nanozyme for colorimetric PPi detection. Copied with permission [101]. Copyright 2019, Royal Society of Chemistry. | |
To obtain the well-controlled nanoprobes with better sensing performance, such metal ions induced nanozymes aggregation was re-creation by converting it into the quantum dots (QDs) system for observing the corresponding adjustable properties and improved performance of QDs nanozymes. As displayed in Fig. 5B, Xia et al. found that Fe3+ can specifically induce an aggregation effect for MoS2 quantum dots (MoS2 QDs) [101]. Further observations suggested that the peroxidase-like activity of MoS2 QDs nanozymes can be precisely regulated through controlling their existed states of aggregation and dispersion in solution. Interestingly, Fe3+-induced aggregation based enhancement of MoS2 QDs nanozymes activity exhibited an obvious decreased performance in the presence of pyrophosphate (PPi) due to the strong interaction between PPi and Fe3+, which further induces the formation of MoS2 QDs dispersions converted from their aggregates. Based on this finding, a reliable colorimetric detection method for PPi was successfully developed based on the Fe3+-induced AIAE-nanozymes system.
Except for metal ions, some small organic molecules also were proposed to induce the formation of aggregated nanozymes, leading to the significant enhancement of the catalytic activity for nanozymes. Such an aggregation effect may also provide a simple and effective route to fabricate reliable and simple detection tools for molecule recognition. For instance, Ni et al. found that melamine can specifically induce the aggregation of gold nanoparticles (AuNPs) nanozymes owing to the good conjugation between melamine and AuNPs [88]. As a result, the ability of bare AuNPs to catalyze colorimetric reaction of the TMB-H2O2 system was significantly improved due to the formation of AIAE-nanozymes. It is seen such AIAE-nanozymes further exhibited a melamine concentration-dependent optical change with sensitive color readout, as shown in Fig. 6A. Similar to this finding, Deng et al. also found that the peroxidase-like activity of Cu2-xSe nanoparticles (Cu2-xSe NPs) can be significantly enhanced when they are aggregated [102]. By using sodium polystyrene sulfonate (SPS) as a modifier and stabilizer, SPS capped Cu2-xSe NPs (Cu2-xSe@SPS NPs) were successfully prepared and used to conjugate with melamine. The resultantly formed Cu2-xSe@SPS NPs aggregates exhibited a significant acceleration effect for Cu2-xSe nanaozyme activity. As a result, the obtained AIAE-nanozymes further catalyzed the TMB-H2O2 colorimetric reaction to produce a sensitive color response (Fig. 6B). Based on these findings, low cost, sensitive and simple method for sensing melamine targets based on AIAE-nanozymes system without the assistance of expensive instruments and complicated modification was successfully developed.

|
Download:
|
| Fig. 6. Schematic illustration of melamine-induced aggregation based activity enhancement of (A) AuNPs nanozyme for colorimetric melamine detection. Copied with permission [88]. Copyright 2014, Elsevier B.V. Schematic illustration of and (B) Cu2-xSe NPs nanozyme for colorimetric melamine sensing. Copied with permission [102]. Copyright 2016, Royal Society of Chemistry. | |
The above efforts not only demonstrate the new concept of AIAE-nanozymes design and their corresponding colorimetric sensing application toward both metal ions and organic molecules, but also indicate that AIAE-nanozymes can provide new insights into developing novel sensing strategy by using nanozymes activity modulated systems. So there are good reasons to believe that AIAE-nanozymes can effectively accelerate the development of nanozymes-based colorimetric sensors. In addition, the superior advantages of the simple principle and easy operation of the recent AIAE-nanozymes system exhibit more valuable in the design of very suitable nanozymes-based platform for disease diagnosis and therapy fields.
Though lots of inorganic nanozymes not only exhibit high selectivity and catalytic efficiency but also harbor well-designed biocompatibility and biodegradability, the biosafety issues of them existed in the in vivo applications further limit their practical utilization [103, 104]. Therefore, it is more significant to develop organic-derived nanozymes with good biocompatibility and biodegradability. But the low delivery efficiency and the loss of enzyme activity further limit the implementation of organic-derived nanozymes in disease diagnosis and therapy [105, 106]. Taking the advantages of AIAE-nanozymes system with effective aggregation-induced modulating performance, He et al. recently developed an ultrasmall organic nanozyme with more reductive and catalytic sites to treat ROS-related diseases in the central nervous system [89]. The good ROS-induced aggregation effect endows the highly effective multienzyme-like activities for the obtained organic-derived nanozymes in the ROS-rich environment, and therefore provides a solution for ROS-related brain injury.
As a short summary, the recently developed AIAE-nanozymes systems have exhibited more precise, simple and effective modulation performance for nanozymes activity, depending on an aggregation-induced strategy that encouraged from traditional SPR and AIE principles. Furthermore, with the appropriate introduction of the target analyte, the resultant AIAE-nanozymes demonstrated more powerful and potential applications in nanozymes based colorimetric sensing field. We also have reason to believe that AIAE-nanozymes can be further extended into the significant exposure of other readout based sensing applications.
5. Nanozymes-based colorimetric sensors based on aggregation-induced activity inhibitionDifferent from the aggregation-enhanced nanozymes activity, some analyte-induced formation of nanozymes aggregation exhibit an obvious inhibition effect for the catalytic activity of nanozymes. As an AIAI-nanozymes mode, though the catalytic activity of nanozymes usually is demonstrated with an inhibited performance, but which can further provide an alternative route for fabricating the selective and sensitive colorimetric method by providing the color readout with a dramatic "signal-off" feature. It therefore may advance the development of the nanozymes-based colorimetric sensors as the new strategy for nanozymes activity regulation.
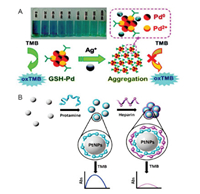
Recent efforts have suggested that different target analytes explored such AIAI-nanozymes properties, such as metal ions [107], polysaccharide molecules, organic molecules and amino acids. Fu and Li selected Ag+ as a model target and investigated its effect on the catalytic activity modulation of Glutathione (GSH)-capped Pd nanoparticles (PdNPs) nanozymes [107]. The obtained observations suggested that Ag+ ions can directly and selectively bind to GSH-capped PdNPs through metallophilic interactions, resulting in a significant aggregation of PdNPs. The peroxidase mimicking activity of PdNPs was greatly inhibited by this Ag+ content-dependent aggregation-induced effect. As a result, a sensitive and simple aggregation-induced nanozymes based colorimetric detection method with a significant "signal-off" color readout for Ag+ was successfully developed (Fig. 7A).
|
Download:
|
| Fig. 7. Schematic illustration of (A) Ag+-induced aggregation based activity inhibition of PdNPs nanozyme for colorimetric Ag+ determination. Copied with permission [107]. Copyright 2015, Royal Society of Chemistry. Schematic illustration of (B) Heparin-induced aggregation based activity inhibition of PtNPs nanozyme for colorimetric heparin detection. Copied with permission [108]. Copyright 2017, Elsevier B.V. | |
For different nanozymes substrates, the appropriate target analyte can be selected and used for tunning the catalytic performance of nanozymes to obtain AIAI-nanozymes and the resultant colorimetric detection method. Following on this thought, the key role to successfully obtain an AIAI-nanozymes relying on the unique interaction between nonozymes surface and target molecules were attracted great attention. In this regard, different target molecules were proposed to firstly induce the aggregation of nanozymes, and further to investigate the performance of the obtained AIAI-nanozymes.
Heparin as a typical polysaccharide in the biological system, plays a key role in the regulation of some physiological and pathological processes [96]. It therefore is important for developing a more rapid, sensitive and reliable colorimetric detection tool to monitor heparin concentrations in the biological system. You et al. found that citrate-capped platinum nanoparticles (PtNPs) have good oxidase mimetics and could catalyze the oxidation of organic substrates to produce colored products with a sensitive color readout response [108]. To achieve an AIAI-nanozymes based colorimetric detection tool, they used protamine as the modifier to prepare the protamine-modified PtNPs nanozymes through the direct adsorption between protamine and citrate that capped on the PtNPs surface. As shown in Fig. 7B, such design further enables the effective PtNPs aggregation in the presence of heparin and induces the inhibition of PtNPs nanozyme activity. Based on these observations, they successfully developed a sensitive and portable AIAI-nanozymes based colorimetric tool for heparin detection.
On the design of AIAI-nanozymes and the fabrication of their resultant colorimetric detection tools, some organic molecules also attracted great interest from researchers owing to their rich reaction types in different chem/biosystems. Wang et al. prepared a protein conjugated AuNCs with a good peroxidase mimic activity [109]. The subsequent investigation indicated that the addition of tea polyphenols (TPs) in the AuNCs nanozyme system can further induce the formation of aggregated AuNCs. Due to this reason, it thus exhibits a dramatic inhibition for AuNCs nnaozymes activity and results in a sensitive TPs-content dependent color response by using TMB-H2O2 colorimetric reaction (Fig. 8A). The observed colorimetric sensing performance may be attributed to the unique interaction between the TPs target and the modifier of AuNCs nanozyme surface, which further leads to the significant nanozyme aggregation and the resultant target-dependent color readout based response.

|
Download:
|
| Fig. 8. Schematic diagram of (A) TPs-induced aggregation based activity inhibition of AuNCs nanozyme for colorimetric TPs sensing. Copied with permission [109]. Copyright 2015, Royal Society of Chemistry. Schematic diagram of (B) cysteine-induced aggregation based activity inhibition of AuNPs nanozyme for colorimetric cysteine detection. Copied with permission [98]. Copyright 2016, Royal Society of Chemistry. | |
Above example both on metal ion and organic molecules induced nanozymes aggregation indicate that the appropriate reaction system can help us to achieve AIAI-nanozymes. It also can break the limitation of nanozymes in the practical application as the signal amplifier with poor abilities of target recognition. As far as the selectivity promotion for nanozymes-based colorimetric tools, the modulation of nanozymes activity such as AIAE-nanozymes and AIAI-nanozymes have been demonstrated to be more useful due to the specific interaction and recognition between target and nanozymes surface.
Except for the smart design using specific interaction between metal ions or organic molecules and the corresponding nanozymes surface, amino acids recently were also proposed to fabricate the AIAI-nanozymes system. Because amino acids possess some active sites to selectively combine with the given modifier on the nanozymes surface, which can be applied to form nanozyme aggregation, resulting in the successful biomolecules-based modulation for nanozymes activity. Li et al. explored that cysteine can effectively induce the aggregation of kiwi juice-prepared AuNPs [98]. The obtained aggregated AuNPs nanozyme exhibited an obviously inhibited activity over the dispersive AuNPs system. Such amino acids-based AIAI-nanozymes provided a simple and practical route for fabricating the colorimetric detection tool for the detection of cysteine (Fig. 8B). On the other hand, amino acids with different structures and active groups themselves may provide more chances to directly regulate the activity of nanozymes via absorbing on the nanozyme surface. It further opens the door for directly colorimetric sensing them through the newly developing AIAI-nanozymes or AIAE-nanozymes.
As a conclusion in AIAI-nanozymes based colorimetric methods, recent efforts have shown more potential in the applications of nanozymes related analysis and testing. In addition, such type of nanozymes with suppressed activity theoretically can be easily obtained by using various large-size biomolecules or long-chain biomolecules such as DNA and proteins, which may be attributed to the good absorbing effect of nanozymes toward these molecules and the resultant decrease on the surface area and active sites of nanozymes. Therefore, it further allows the potential prospect of biomolecules-based strategies for fabricating AIAI-nanozymes and expanding their practical colorimetric sensing applications.
6. Summary and future perspectivesIn this review, we summarize the recent developments in aggregation-induced nanozymes based colorimetric bio/chemosensors. The findings from lots of recent efforts on aggregation-induced enhancement and aggregation-induced inhibition for nanozymes activity (AIAE-nanozyme and AIAI-nanozymes) exhibited more advantages in the fabrication and practical application of the corresponding nanozymes based colorimetric sensors. First, as a typical label-free chromogenic substrate, aggregation-induced nanozymes exhibit simple yet diversified routes to regulate their mimic activities, resulting in a significant acceleration for nanozymes-based colorimetric detection tools. Second, similar to conventional SPR-format based colorimetric sensors, both AIAE-nanozymes and AIAI-nanozymes in aggregation-induced nanozymes family have low technical and instrumental demands, further leading to the successful simplification on nanozymes-based colorimetric methods. Third, the newly developed aggregation-induced modulation for nanozymes activity sometimes can break the limitation of nanozymes in the special application fields such as in vivo samples and biological detecting events at neutral pH. It thus is very important for expanding the practical application of nanozymes-based colorimetric assays.
Though great efforts have been made in the development of aggregation-induced nanozymes and the advances of their corresponding colorimetric detection tools, some critical issues we think still need to be considered and resolved to further broaden the application of such nanozymes systems. These critical issues may be attracted the great attention as the future developing directions in this field.
(ⅰ) The existing strategies for aggregation-induced nanozymes usually are developed relying on the unique interaction between the active groups that capped on the nanozymes surface and the target species. Though the corresponding detection events for target analytes have recently been developed successfully, the regulation principles of such systems for enhancing or inhibiting nanozymes activity need to be further investigated deeply. For example, AuNPs nanozyme may present AIAE- or AIAI-nanozymes features in different cases. The deep and comprehensive understanding of the same nanozymes substrate would greatly help us to develop more potential sensing platforms, and to further expand the practical application of such newly explored aggregation-induced nanozymes.
(ⅱ) To further advance the practical application of aggregation-induced nanozymes, more particular efforts with good synthesis reproducibility we think should be devoted in the future, not only to prepare standard nanozymes-based analytical materials, but also to build more practical colorimetric devices with standardized response performance. For example, it is very promising to develop ultrasmall nanozymes substrate with aggregation-induced controllable activities through some standard methods by using different chemical or physical technologies, so as to treat diseases well in vivo applications such as cancer therapy, radiation protection and neuronal injury recovery.
(ⅲ) The color readout in all aggregation-induced nanozymes in the developed methods are generated on the basis of some specific chromogenic reaction that catalyzed by aggregation-induced nanozymes, such as for the TMB, OPD and ABTS oxidation related reaction systems. Though some superior advantages such as simple, cost-effective, facile and portable have been demonstrated fully, the enhanced sensing performance and promoted selectivity based on effective amplification and signal conversion are scarce and need to be strengthened further. For instance, using such activity controllable nanozymes to build temperature readout-based detection tools, could effectively break the low-resolution limitation of color change-based readout in the traditional nanozymes-based colorimetric methods. For another example, using aggregation-induced nanozymes system to catalyze gas-generation reaction for achieving pressure readout-based sensors. Both temperature and pressure signals as the simple, portable and powerful sensing readout would be more potential for expanding the practical application of aggregation-induced nanozymes.
Overall, as a new strategy for nanozymes activity modulation, aggregation-induced nanozymes demonstrated more advantages in the fabrication of nanozymes-based colorimetric sensors. Though great advances and remarkable progresses have been made, it still remains plenty of room for the promotion and development of aggregation-induced nanozymes as the next frontier of nanozymes modulation and regulation.
Declaration of competing interestThe authors report no declarations of interest.
AcknowledgmentsThe authors thank the National Natural Science Foundation of China (Nos. 21765013, 21665023), Key Talent Project of Gansu Province, "Feitian Scholar" Program of Gansu Province, and the Research Projects of Universities in Gansu Province (No. 2017A-115) for financially supporting this research.
| [1] |
K. Saha, S.S. Agasti, C. Kim, X. Li, V.M. Rotello, Chem. Rev. 112 (2012) 2739-2779. DOI:10.1021/cr2001178 |
| [2] |
T. Wei, T. Dong, Z. Wang, et al., J. Am. Chem. Soc. 137 (2015) 8880-8883. DOI:10.1021/jacs.5b04348 |
| [3] |
W. Chansuvarn, T. Tuntulani, A. Imyim, TrAC-Trend Anal. Chem. 65 (2015) 83-96. DOI:10.1016/j.trac.2014.10.013 |
| [4] |
S.K. Kailasa, J.R. Koduru, M.L. Desai, et al., TrAC-Trend Anal. Chem. 105 (2018) 106-120. DOI:10.1016/j.trac.2018.05.004 |
| [5] |
Z.Y. Dong, D.W. Zhang, X.Z. Jiang, H. Li, G.H. Gao, Chin. Chem. Lett. 24 (2013) 688-690. DOI:10.1016/j.cclet.2013.04.048 |
| [6] |
X.Q. Chen, F. Wang, J.Y. Hyun, et al., Chem. Soc. Rev. 45 (2016) 2976-3016. DOI:10.1039/C6CS00192K |
| [7] |
S. Lee, K.K.Y. Yuen, K.A. Jolliffe, J. Yoon, Chem. Soc. Rev. 44 (2015) 1749-1762. DOI:10.1039/C4CS00353E |
| [8] |
H.A. Ho, A. Najari, M. Leclerc, Acc. Chem. Res. 41 (2008) 168-178. DOI:10.1021/ar700115t |
| [9] |
R. Wilson, Chem. Soc. Rev. 37 (2008) 2028-2045. DOI:10.1039/b712179m |
| [10] |
X. Fu, L. Chen, J. Choo, Anal. Chem. 89 (2017) 124-137. DOI:10.1021/acs.analchem.6b02251 |
| [11] |
T. Wu, Y.F. Li, C.Z. Huang, Chin. Chem. Lett. 20 (2009) 611-614. DOI:10.1016/j.cclet.2009.01.024 |
| [12] |
Y. Zhang, Y.J. Zhang, X.D. Xia, et al., Chin. Chem. Lett. 24 (2013) 1053-1058. DOI:10.1016/j.cclet.2013.07.021 |
| [13] |
R. Breslow, L.E. Overman, J. Am. Chem. Soc. 92 (1970) 1075-1077. DOI:10.1021/ja00707a062 |
| [14] |
J. Wu, X. Wang, Q. Wang, et al., Chem. Soc. Rev. 48 (2019) 1004-1076. DOI:10.1039/C8CS00457A |
| [15] |
Y. Lin, J. Ren, X. Qu, Acc. Chem. Res. 47 (2014) 1097-1105. DOI:10.1021/ar400250z |
| [16] |
L. Gao, J. Zhuang, L. Nie, et al., Nat. Nanotechnol. 2 (2007) 577-583. DOI:10.1038/nnano.2007.260 |
| [17] |
Y. Huang, J. Ren, X. Qu, Chem. Rev. 119 (2019) 4357-4412. DOI:10.1021/acs.chemrev.8b00672 |
| [18] |
Y. Zhou, B. Liu, R. Yang, J. Liu, Bioconjugate Chem. 28 (2017) 2903-2909. DOI:10.1021/acs.bioconjchem.7b00673 |
| [19] |
Y. Lin, J. Ren, X. Qu, Adv. Mater. 26 (2014) 4200-4217. DOI:10.1002/adma.201400238 |
| [20] |
P. Pengo, S. Polizzi, L. Pasquato, P. Scrimin, J. Am. Chem. Soc. 127 (2005) 1616-1617. DOI:10.1021/ja043547c |
| [21] |
W. Luo, C. Zhu, S. Su, et al., ACS Nano 4 (2010) 7451-7458. DOI:10.1021/nn102592h |
| [22] |
X. Zheng, Q. Liu, C. Jing, et al., Angew. Chem. Int. Ed. 50 (2011) 11994-11998. DOI:10.1002/anie.201105121 |
| [23] |
H. Zhang, J. Chen, Y. Yang, et al., Anal. Chem. 91 (2019) 5004-5010. DOI:10.1021/acs.analchem.8b04779 |
| [24] |
A. Asati, S. Santra, C. Kaittanis, S. Nath, J.M. Perez, Angew. Chem. Int. Ed. 48 (2009) 2308-2312. DOI:10.1002/anie.200805279 |
| [25] |
T. Pirmohamed, J.M. Dowding, S. Singh, et al., Chem. Commun. 46 (2010) 2736-2738. DOI:10.1039/b922024k |
| [26] |
F. Natalio, R. André, A.F. Hartog, et al., Nat. Nanotechnol. 7 (2012) 530-535. DOI:10.1038/nnano.2012.91 |
| [27] |
X. Sun, S. Guo, C.S. Chung, W. Zhu, S. Sun, Adv. Mater. 25 (2013) 132-136. DOI:10.1002/adma.201203218 |
| [28] |
X. Sun, S. Guo, Y. Liu, S. Sun, Nano Lett. 12 (2012) 4859-4863. DOI:10.1021/nl302358e |
| [29] |
R. Wolfenden, M.J. Snider, Acc. Chem. Res. 34 (2001) 938-945. DOI:10.1021/ar000058i |
| [30] |
P. Weerathunge, R. Ramanathan, R. Shukla, T.K. Sharma, V. Bansal, Anal. Chem. 86 (2014) 11937-11941. DOI:10.1021/ac5028726 |
| [31] |
L.Y. Jin, Y.M. Dong, X.M. Wu, G.X. Cao, G.L. Wang, Anal. Chem. 87 (2015) 10429-10436. DOI:10.1021/acs.analchem.5b02728 |
| [32] |
M. Liang, X. Yan, Acc. Chem. Res. 52 (2019) 2190-2200. DOI:10.1021/acs.accounts.9b00140 |
| [33] |
X. Wang, Y. Hu, H. Wei, Inorg. Chem. Front. 3 (2016) 41-60. DOI:10.1039/C5QI00240K |
| [34] |
B. Liu, Z. Sun, P.J.J. Huang, J. Liu, J. Am. Chem. Soc. 137 (2015) 1290-1295. DOI:10.1021/ja511444e |
| [35] |
J. Wu, X. Wang, Q. Wang, et al., Chem. Soc. Rev. 48 (2019) 1004-1076. DOI:10.1039/C8CS00457A |
| [36] |
Z. Chen, J.J. Yin, Y.T. Zhou, et al., ACS Nano 6 (2012) 4001-4012. DOI:10.1021/nn300291r |
| [37] |
A. Asati, C. Kaittanis, S. Santra, J.M. Perez, Anal. Chem. 83 (2011) 2547-2553. DOI:10.1021/ac102826k |
| [38] |
J.W. Lee, H.J. Jeon, H.J. Shin, J.K. Kang, Chem. Commun. 48 (2012) 422-424. DOI:10.1039/C1CC15725F |
| [39] |
C. Ge, G. Fang, X. Shen, et al., ACS Nano 10 (2016) 10436-10445. DOI:10.1021/acsnano.6b06297 |
| [40] |
N. Puvvada, P.K. Panigrahi, D. Mandal, A. Pathak, RSC Adv. 2 (2012) 3270-3273. DOI:10.1039/c2ra01081j |
| [41] |
A. Ghosh, S. Basak, B.H. Wunsch, R. Kumar, F. Stellacci, Angew. Chem. Int. Ed. 50 (2011) 7900-7905. DOI:10.1002/anie.201101821 |
| [42] |
Y. Hu, X.J. Gao, Y. Zhu, et al., Chem. Mater. 30 (2018) 6431-6439. DOI:10.1021/acs.chemmater.8b02726 |
| [43] |
D.A. Giljohann, C.A. Mirkin, Nature 462 (2009) 461-464. DOI:10.1038/nature08605 |
| [44] |
D. Duan, K. Fan, D. Zhang, et al., Biosens. Bioelectron. 74 (2015) 134-141. DOI:10.1016/j.bios.2015.05.025 |
| [45] |
J. Xi, C. Xie, Y. Zhang, et al., ACS Appl. Mater. Inter. 8 (2016) 22563-22573. DOI:10.1021/acsami.6b05561 |
| [46] |
F. Tian, J. Zhou, B. Jiao, Y. He, Nanoscale 11 (2019) 9547-9555. DOI:10.1039/C9NR02872B |
| [47] |
L. Huang, K. Chen, W. Zhang, et al., Sens. Actuators B: Chem. 269 (2018) 79-87. |
| [48] |
X. Zhang, D. Wu, X. Zhou, et al., TrAC-Trend Anal. Chem. 121 (2019) 115668. DOI:10.1016/j.trac.2019.115668 |
| [49] |
Y. Meng, W. Li, X. Pan, G.M. Gadd, Environ. Sci.: Nano 7 (2020) 1305-1318. DOI:10.1039/C9EN01089K |
| [50] |
P. Borthakur, M.R. Das, S. Szunerits, R. Boukherroub, ACS Sustain. Chem. Eng. 7 (2019) 16131-16143. DOI:10.1021/acssuschemeng.9b03043 |
| [51] |
S.A. El-Safty, M.A. Shenashen, S.A. El-Safty, TrAC-Trend Anal. Chem. 38 (2012) 98-115. DOI:10.1016/j.trac.2012.05.002 |
| [52] |
C.L. Nehl, J.H. Hafner, J. Mater. Chem. 18 (2008) 2415-2419. DOI:10.1039/b714950f |
| [53] |
E. Ozbay, Science 311 (2006) 189-193. DOI:10.1126/science.1114849 |
| [54] |
C. Noguez, J. Phys. Chem. C 111 (2007) 3806-3819. DOI:10.1021/jp066539m |
| [55] |
X. Ma, H. Sun, Y. Wang, X. Wu, J. Zhang, Nano Energy 53 (2018) 932-939. DOI:10.1016/j.nanoen.2018.09.042 |
| [56] |
E. Hutter, J.H. Fendler, Adv. Mater. 16 (2004) 1685-1706. DOI:10.1002/adma.200400271 |
| [57] |
K.M. Mayer, J.H. Hafner, Chem. Rev. 111 (2011) 3828-3857. DOI:10.1021/cr100313v |
| [58] |
M. Li, S.K. Cushing, N. Wu, Analyst 140 (2015) 386-406. DOI:10.1039/C4AN01079E |
| [59] |
X. Ma, S. He, B. Qiu, et al., ACS Sens. 4 (2019) 782-791. DOI:10.1021/acssensors.9b00438 |
| [60] |
S. Szunerits, R. Boukherroub, Chem. Commun. 48 (2012) 8999-9010. DOI:10.1039/c2cc33266c |
| [61] |
P. Singh, Sens. Actuators B: Chem. 229 (2016) 110-130. DOI:10.1016/j.snb.2016.01.118 |
| [62] |
H. Huang, C. He, Y. Zeng, et al., Biosens. Bioelectron. 24 (2009) 2255-2259. DOI:10.1016/j.bios.2008.10.013 |
| [63] |
J. Luo, Z. Xie, J.W.Y. Lam, et al., Chem. Commun. 18 (2001) 1740-1741. |
| [64] |
J.M. Delente, D. Umadevi, S. Shanmugaraju, et al., Chem. Commun. 56 (2020) 2562-2565. DOI:10.1039/C9CC08457F |
| [65] |
M. Baglan, S. Atılgan, Chem. Commun. 49 (2013) 5325-5327. DOI:10.1039/c3cc42238k |
| [66] |
V. Kumar, P. Kumar, A. Pournara, K. Vellingiri, K.H. Kim, TrAC-Trend Anal. Chem. 106 (2018) 84-115. DOI:10.1016/j.trac.2018.07.003 |
| [67] |
H. Rao, X. Xue, H. Wang, Z. Xue, J. Mater. Chem. C 7 (2019) 4610-4621. DOI:10.1039/C9TC00757A |
| [68] |
Q. Feng, Y. Li, L. Wang, et al., Chem. Commun. 52 (2016) 3123-3126. DOI:10.1039/C5CC10423H |
| [69] |
S. Zhang, J.M. Yan, A.J. Qin, J.Z. Sun, B.Z. Tang, Chin. Chem. Lett. 24 (2013) 668-672. DOI:10.1016/j.cclet.2013.05.014 |
| [70] |
R. Pautler, E.Y. Kelly, P.J.J. Huang, et al., ACS Appl. Mater. Inter. 5 (2013) 6820-6825. DOI:10.1021/am4018863 |
| [71] |
D. Yang, M. Fa, L. Gao, et al., Nanotechnology 29 (2018) 385101. DOI:10.1088/1361-6528/aacf86 |
| [72] |
C. Xu, Z. Liu, L. Wu, J. Ren, X. Qu, Adv. Funct. Mater. 24 (2014) 1624-1630. DOI:10.1002/adfm.201301649 |
| [73] |
G. Bülbül, A. Hayat, S. Andreescu, Adv. Healthc. Mater. 5 (2016) 822-828. DOI:10.1002/adhm.201500705 |
| [74] |
Q. Luan, K. Zhou, H. Tan, D. Yang, X. Yao, Biosens. Bioelectron. 26 (2011) 2473-2477. DOI:10.1016/j.bios.2010.10.035 |
| [75] |
L. Zhan, Z. Chen, S. Gong, et al., Angew. Chem. Int. Ed. 58 (2019) 17651-17655. DOI:10.1002/anie.201910719 |
| [76] |
C.T. Lai, J.L. Hong, J. Phys. Chem. B 114 (2010) 10302-10310. DOI:10.1021/jp1012297 |
| [77] |
R. Hu, A. Qin, B.Z. Tang, Prog. Polym. Sci. 100 (2020) 101176. DOI:10.1016/j.progpolymsci.2019.101176 |
| [78] |
L. Yan, Y. Zhang, B. Xu, W. Tian, Nanoscale 8 (2016) 2471-2487. DOI:10.1039/C5NR05051K |
| [79] |
Z. Luo, X. Yuan, Y. Yu, et al., J. Am. Chem. Soc. 134 (2012) 16662-16670. DOI:10.1021/ja306199p |
| [80] |
C. Tang, H. Feng, Y. Huang, Z. Qian, Anal. Chem. 89 (2017) 4994-5002. DOI:10.1021/acs.analchem.7b00319 |
| [81] |
B. Tan, H. Zhao, W. Wu, et al., Nanoscale 9 (2017) 18699-18710. DOI:10.1039/C7NR05541B |
| [82] |
S. Singh, T. Dosani, A.S. Karakoti, et al., Biomaterials 32 (2011) 6745-6753. DOI:10.1016/j.biomaterials.2011.05.073 |
| [83] |
B. Liu, J. Liu, Chem. Commun. 50 (2014) 8568-8570. DOI:10.1039/C4CC03264K |
| [84] |
F. Wang, J. Liu, Small 10 (2014) 3927-3931. DOI:10.1002/smll.201400850 |
| [85] |
Y. Zhao, H. Li, A. Lopez, H. Su, J. Liu, ChemBioChem (2020), doi:http://dx.doi.org/10.1002/cbic.202000049.
|
| [86] |
E. Ögüt, Ç. Kip, B. Gökçal, A. Tuncel, J. Colloid. Interf. Sci. 550 (2019) 90-98. DOI:10.1016/j.jcis.2019.04.089 |
| [87] |
H. Liao, G. Liu, Y. Liu, et al., Chem. Commun. 53 (2017) 10160-10163. DOI:10.1039/C7CC05409B |
| [88] |
P. Ni, H. Dai, Y. Wang, et al., Biosens. Bioelectron. 60 (2014) 286-291. DOI:10.1016/j.bios.2014.04.029 |
| [89] |
H. He, X. Shi, J. Wang, et al., ACS Appl. Mater. Inter. 12 (2020) 209-216. DOI:10.1021/acsami.9b17509 |
| [90] |
P. An, X. Xue, H. Rao, et al., Anal. Chim. Acta 1125 (2020) 114-127. DOI:10.1016/j.aca.2020.05.047 |
| [91] |
M. Gao, P. An, H. Rao, et al., Analyst 145 (2020) 1279-1287. DOI:10.1039/C9AN01956A |
| [92] |
M. Luo, X. Xue, H. Rao, et al., Sens. Actuators B: Chem. 309 (2020) 127707. DOI:10.1016/j.snb.2020.127707 |
| [93] |
X. Xue, M. Gao, H. Rao, et al., Anal. Chim. Acta 1105 (2020) 197-207. DOI:10.1016/j.aca.2020.01.049 |
| [94] |
H. Wei, E. Wang, Chem. Soc. Rev. 42 (2013) 6060-6093. DOI:10.1039/c3cs35486e |
| [95] |
Y. Huang, J. Ren, X. Qu, Chem. Rev. 119 (2019) 4357-4412. DOI:10.1021/acs.chemrev.8b00672 |
| [96] |
L. Bergamaschini, E. Rossi, C. Vergani, M.G. De Simoni, Sci. World J. 9 (2009) 891-908. DOI:10.1100/tsw.2009.100 |
| [97] |
H. Jiang, W. Xu, Q. Chen, Food Chem. 319 (2020) 126584. DOI:10.1016/j.foodchem.2020.126584 |
| [98] |
R.S. Li, H. Liu, B.B. Chen, et al., Anal. Methods 8 (2016) 2494-2501. DOI:10.1039/C6AY00367B |
| [99] |
W. Xu, D. Wang, B.Z. Tang, Angew. Chem. Int. Ed. (2020), doi: http://dx.doi.org/10.1002/anie.202005899.
|
| [100] |
Q. Lu, X. Chen, D. Liu, et al., Microchim. Acta 186 (2019) 72. DOI:10.1007/s00604-018-3181-z |
| [101] |
W. Xia, P. Zhang, W. Fu, L. Hu, Y. Wang, Chem. Commun. 55 (2019) 2039-2042. DOI:10.1039/C8CC09799B |
| [102] |
S.Q. Deng, H.Y. Zou, J. Lan, C.Z. Huang, Anal. Methods 8 (2016) 7516-7521. DOI:10.1039/C6AY02275H |
| [103] |
K. Fan, J. Xi, L. Fan, et al., Nat. Commun. 9 (2018) 1440. DOI:10.1038/s41467-018-03903-8 |
| [104] |
Z. Wang, Y. Zhang, E. Ju, et al., Nat. Commun. 9 (2018) 3334. DOI:10.1038/s41467-018-05798-x |
| [105] |
S. Wang, W. Chen, A.L. Liu, et al., ChemPhysChem 13 (2012) 1199-1204. DOI:10.1002/cphc.201100906 |
| [106] |
C.P. Liu, T.H. Wu, Y.L. Lin, et al., Small 12 (2016) 4127-4135. DOI:10.1002/smll.201503919 |
| [107] |
Y. Fu, H. Zhang, S. Dai, et al., Analyst 140 (2015) 6676-6683. DOI:10.1039/C5AN01103E |
| [108] |
J.G. You, Y.W. Liu, C.Y. Lu, W.L. Tseng, C.J. Yu, Biosens. Bioelectron. 92 (2017) 442-448. DOI:10.1016/j.bios.2016.10.082 |
| [109] |
S. Wang, P. Liu, Y. Qin, Z. Chen, J. Shen, Sens. Actuators B: Chem. 223 (2016) 178-185. DOI:10.1016/j.snb.2015.09.058 |
 2021, Vol. 32
2021, Vol. 32 

