b School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002, China;
c College of Animal Science and Technology, Yangzhou University, Yangzhou 225009, China;
d Department of Applied Chemistry, Faculty of Engineering, Kyushu Institute of Technology, Kitakyushu 804-8550, Japan;
e Sichuan Selewood Technology Company Limited, Chengdu 610218, China;
f College of Veterinary Medicine, Yangzhou University, Yangzhou 225009, China
Chalcogen elements have very wide application scopes for their unique bio- and chemical-activities [1-3]. In the field, investigations on selenium chemistry are unfolding with comprehensive application scopes [4-21]. In our cases, we recently began to pay attention to the application of the selenium-containing chemicals in agriculture [22]. In comparison with traditional chemical pesticides, there are many advantages in using the selenium-containing substitutes. As an essential element for human beings that can be metabolized in the body, selenium is safe to the environment [23]. Moreover, the tolerance of selenium residues is better than that of transition metals according to the latest revision of the USP's New Standard and Method for Elemental Impurities Control in 2017 [24]. Since China possesses rich selenium resources, the price of selenium is relatively low and the cost for producing the related selenium-containing chemicals is acceptable if the preparations are controlled in limited steps of reactions. Despite the bio-effects, the use of selenium-containing chemicals in agriculture may also facilitate the selenium-enrichment in the products, i.e., they have doubled beneficial effects on both disease control and seleniumenriched food production.
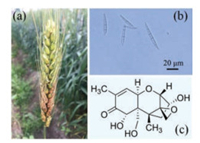
On the other hand, wheat scab disease (Fig. 1a) is one of the major wheat diseases caused by Fusarium graminearum species complex (F.g.) (Fig. 1b) [25]. It is prevalent in the wheat production area of the middle and lower reaches of the Yangtze River, the east of spring wheat area in Northeast China and the wheat production area of South China, and can cause up to ca.10%–80% yield loss. One of the major detriments of this disease is the generation of mycotoxin deoxynivalenol (DON) (Fig. 1c) in wheat grains, which is one of dangerous secondary metabolites threatening human and animal health [26]. Selenium treatment was able to inhibit Fusarium growth and mycotoxin production [27, 28], but the present techniques employ the inorganic selenium compounds such as Na2SeO3 and Na2SeO4, which are highly toxic and not safe to the environment [29]. Recently, we found that the relatively safe selenized glucose could inhibit DON toxin generation caused by the F.g. Herein, we wish to report our findings.
|
Download:
|
| Fig. 1. Disease symptoms of wheat scab disease (a), the micrograph of F.g. (b) and the chemical structure of mycotoxin DON (c). | |
Selenium powder was used as the selenium source for its low cost and stability. Treating selenium powder with NaBH4 led to NaHSe, a very strong nucleophile that could react with carbonyls through nucleophilic addition. The selenization reagent (NaHSe) was prepared in situ and used without separation. Its addition with glucose occurred in ethanol medium and produced the selenized glucose smoothly [30, 31]. Different from the synthesis of organo-selenium compounds such as selenocysteine and selenomethio-nine [32, 33], in the protocol, the bio-compatible glucose was used as the carrier for the low valent organoselenium and the selenium uploading process required only one-step reaction [30, 31]. Therefore, the production cost can be significantly reduced when compared with traditional organic synthesis and product separation, in which multiple steps are involved. The selenized glucose has already been commercialized and can be used in the seleniumenriched fertilizer production [34]. A series of selenized glucoses with different selenium contents could be prepared by adjusting the molar ratios of the selenium powder vs. glucose (Table 1). Details for the preparation of the Se/C materials were given in Supporting information. The selenium contents were determined by inductively coupled plasma-mass spectrometry (ICP-MS). The use of 1 mol% of selenium (vs. glucose) led to Se-glu (1%) with 0.11% weight content of selenium (Table 1, entry 1). Se-glu (10%) and Seglu (20%) were also prepared via similar methods and the weight contents of selenium were 3.23% and 6.57%, respectively (Table 1, entries 2 and 3).
|
|
Table 1 Selenium content of the prepared selenized glucoses.a |
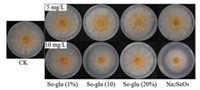
Bio-activities of the selenized glucoses were then tested via F.g. inhibition experiments. Details of the bio-test experiments were described in Supporting information. The F.g. isolate PH-1 was cultured in the trichothecene biosynthesis induction (TBI) solid medium supplemented with a series of selenium-containing compounds, and the diameter (d) of mycelial colony was measured at 96 h after culture, with the F.g. growth diameter (d0) in the TBI medium without supplementation of any form of selenium as a control (CK). The growth inhibition rate of the hyphae was calculated via the equation: Inhibition rate = [(d0-d)/d0]×100%. Photographs of the experiments were depicted in Fig. 2, and the detailed data were summarized in Table 2. Compared with the control (Table 2, entry 1), all of the Se treatments except for the treatment with Se-glu (10%, 5 mg/L) (Table 2, entry 4) delayed the growth of the fungus, and the diameter of the colonies became smaller (Table 2, entries 2, 3, 5–9). The inorganic Na2SeO4 also inhibited the growth of the F.g. (Table 2, entry 8), and showed much higher inhibition rate when supplemented with a doubled dosage (Table 2, entry 9). However, considering its high toxicity, Na2SeO4 was not favorable from the environment-protection viewpoint.
|
Download:
|
| Fig. 2. The mycelial colony on the TBI medium cultured for 96 h treated by the selenized glucoses and inorganic selenium, respectively. | |
|
|
Table 2 The inhibition rate of the F.g. growth by the selenium-containing compounds.a |
DON contents were determined by Triple Quadruopole LC/MS/ MS. In the control sample, DON content was detected to be 35.86 ppb (Table 3, entry 1). By treating with selenium compounds, the generation of DON was completely inhibited, regardless of the types of the selenium reagents (Table 3, entries 2–9). Thus, although the selenized glucoses have weaker inhibitory effects on the F.g. growth in comparison with Na2SeO4 (10 mg/L), they are as good as the inorganic selenium compounds in inhibition of DON production. It is very interesting that, all Se forms (and various dose) were able to inhibit DON production despite of showing different effects on suppression of the pathogen growth. This is possibly due to selenium inhibition of the expression of trichothecene synthetic genes that are responsible for DON production in the pathogen [35], and investigation of the exact mechanism involved is underway. Moreover, toxicity test experiment according to GB 15193.3-2014 showed that the dose of median lethal dose (LD50) of the selenized glucose in mice was 246 mg/kg, which was much safer than Na2SeO4 (LD50 = 1.6 mg/kg). In addition, supplementation of glucose in the medium did not have any effect on DON production by the pathogen [36]. Thus, from the practical viewpoint, the selenized glucose will be more preferable for their relatively lower toxicity than inorganic selenium compounds such as Na2SeO4.
|
|
Table 3 Inhibition of the DON accumulation by the selenium-containing compounds.a |
In conclusion, we found that selenized glucose may inhibit the growth of F.g., and the inhibition rate was not as good as Na2SeO4, but the material could significantly inhibit the generation of DON toxin produced by F.g. This is the first report of using the relatively safe organoselenium compounds to reduce the detriment of wheat scab disease, affording a potential environment-friendly method for using the relatively less toxic material. It is also a breakthrough in the field and will largely expand the application scope of organoselenium chemistry. The work also demonstrates that selenized glucose, the commercialized selenium source for selenium-enriched fertilizer production, may be beneficial for the plant disease control and is a potential doubleeffect reagent in agricultural chemistry [34]. The related experiments on wheat in field environment and continuous investigations on the applications of the selenized glucose are ongoing.
Declaration of competing interestThere is no interest statement to declare.
AcknowledgmentsThis work was supported by the National Key R & D Program: Intergovernmental Key Items for International Scientific and Technological Innovation Cooperation (No. 2018YFE0107700), the open funds of the Key Laboratory of Plant Functional Genomics of the Ministry of Education (No. ML201904), Jiangsu Provincial Six Talent Peaks Project (No. XCL-090), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Appendix A. Supplementary dataSupplementary material related to this article can befound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.06.033.
| [1] |
W.H. Bao, M. He, J.T. Wang, et al., J. Org. Chem. 84 (2019) 6065-6071. DOI:10.1021/acs.joc.9b00178 |
| [2] |
X. Deng, H.E. Cao, C. Chen, et al., Sci. Bull. 64 (2019) 1280-1284. DOI:10.1016/j.scib.2019.07.007 |
| [3] |
M.X. Liu, Y.M. Li, L. Yu, et al., Sci. China Chem. 61 (2018) 294-299. DOI:10.1007/s11426-017-9158-y |
| [4] |
S. Kodama, T. Saeki, K. Mihara, et al., J. Org. Chem. 82 (2017) 12477-12484. DOI:10.1021/acs.joc.7b02276 |
| [5] |
L.H. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30 (2019) 1237-1240. DOI:10.1016/j.cclet.2019.04.033 |
| [6] |
L.H. Lu, S.J. Zhou, W.B. He, et al., Org. Biomol. Chem. 16 (2018) 9064-9068. DOI:10.1039/C8OB02368A |
| [7] |
M. Wang, Q.L. Fan, X.F. Jiang, Org. Lett. 18 (2016) 5756-5759. DOI:10.1021/acs.orglett.6b03078 |
| [8] |
H.E. Cao, R.R. Qian, L. Yu, Catal. Sci. Technol. 10 (2020) 3113-3121. DOI:10.1039/D0CY00400F |
| [9] |
C. Chen, Y.T. Cao, X.X. Wu, et al., Chin. Chem. Lett. 31 (2020) 1078-1082. DOI:10.1016/j.cclet.2019.12.019 |
| [10] |
Y.H. Zheng, A.Q. Wu, Y.Y. Ke, et al., Chin. Chem. Lett. 30 (2019) 937-941. DOI:10.1016/j.cclet.2019.01.012 |
| [11] |
H.E. Cao, M.X. Liu, R.R. Qian, et al., Appl. Organomet. Chem. 33 (2019) e4599. DOI:10.1002/aoc.4599 |
| [12] |
X. Liu, Y.Y. Liang, J.Y. Ji, et al., J. Am. Chem. Soc. 140 (2018) 4782-4786. DOI:10.1021/jacs.8b01513 |
| [13] |
T.T. Wang, X.B. Jing, C. Chen, et al., J. Org. Chem. 82 (2017) 9342-9349. DOI:10.1021/acs.joc.7b01245 |
| [14] |
F. Wang, L. Xu, C. Sun, et al., Chin. J. Org. Chem. 37 (2017) 2115-2118. DOI:10.6023/cjoc201701026 |
| [15] |
X.B. Jing, T.T. Wang, Y.H. Ding, et al., Appl. Catal. A-Gen. 541 (2017) 107-111. DOI:10.1016/j.apcata.2017.05.007 |
| [16] |
G.M. Fang, X.X. Chen, Q.Q. Yang, et al., Chin. Chem. Lett. 29 (2018) 1033-1042. DOI:10.1016/j.cclet.2018.02.002 |
| [17] |
L. Yu, H.E. Cao, X. Zhang, C. Yang, L. Yu, Sustain. Energ. Fuels 4 (2020) 730-736. DOI:10.1039/C9SE00850K |
| [18] |
C. Liu, J.F. Mao, X. Zhang, L. Yu, Catal. Commun. 133 (2020) 105828. DOI:10.1016/j.catcom.2019.105828 |
| [19] |
K.H. Cao, X. Deng, T. Chen, Q.T. Zhang, L. Yu, J. Mater. Chem. A 7 (2019) 10918-10923. DOI:10.1039/C9TA00846B |
| [20] |
X.B. Jing, C.Z. Chen, X. Deng, et al., Appl. Organomet. Chem. 32 (2018) e4332. DOI:10.1002/aoc.4332 |
| [21] |
Y. Wang, L. Yu, B. Zhu, L. Yu, J. Mater. Chem. A 4 (2016) 10828-10833. DOI:10.1039/C6TA02566H |
| [22] |
H.E. Cao, Y.F. Yang, X.J. Chen, et al., Chin. Chem. Lett. 31 (2020) 1887-1889. DOI:10.1016/j.cclet.2020.01.027 |
| [23] |
M.P. Rayman, Lancet 379 (2012) 1256-1268. DOI:10.1016/S0140-6736(11)61452-9 |
| [24] |
US Pharmacopeial convention (USP), USP < 232> Elemental Impurities-Limits. 40-NF 35, First Supplement (2017). https://www.usp.org/sites/default/files/usp/document/ourwork/chemical-medicines/key-issues/232-40-35-1s.pdf.
|
| [25] |
B.A. Summerell, Annu. Rev. Phytopathol. 57 (2019) 323-339. DOI:10.1146/annurev-phyto-082718-100204 |
| [26] |
K. Nesic, S. Ivanovic, V. Nesic, Rev. Environ. Contam. Toxicol. 228 (2014) 101-120. |
| [27] |
B.L. Cheng, Y. Zhang, B. Tong, et al., Biol. Trace Elem. Res. 178 (2017) 147-152. DOI:10.1007/s12011-016-0900-3 |
| [28] |
F.Y. Sun, L. Yang, L. Li, et al., Curr. Biotech. 7 (2017) 433-438. |
| [29] |
Q.H. Wu, X. Wang, E. Nepovimova, et al., Oncotarget 8 (2017) 110708-110726. DOI:10.18632/oncotarget.22800 |
| [30] |
S.N. Chu, H.E. Cao, T. Chen, et al., Catal. Commun. 129 (2019) 105730. DOI:10.1016/j.catcom.2019.105730 |
| [31] |
Y.F. Yang, X. Fan, H.E. Cao, et al., Catal. Sci. Technol. 8 (2018) 5017-5023. DOI:10.1039/C8CY01413B |
| [32] |
J.C. Peeler, J.A. Falco, R.E. Kelemen, et al., ACS Chem. Biol. 15 (2020) 1535-1540. DOI:10.1021/acschembio.0c00147 |
| [33] |
J. Qu, W. Wang, Q. Zhang, S. Li, Biol. Trace Elem. Res. 195 (2020) 205-214. DOI:10.1007/s12011-019-01841-0 |
| [34] |
W.J. Zhou, P.Z. Li, J. Liu, et al., Ind. Eng. Chem. Res. 59 (2020) 10763-10767. DOI:10.1021/acs.iecr.0c01147 |
| [35] |
Y. Chen, H.C. Kistler, Z.H. Ma, Annu. Rev. Phytopathol. 57 (2019) 15-39. DOI:10.1146/annurev-phyto-082718-100318 |
| [36] |
F. Jiao, A. Kawakami, T. Nakajima, FEMS Microbiol. Lett. 285 (2008) 212-219. DOI:10.1111/j.1574-6968.2008.01235.x |
 2020, Vol. 31
2020, Vol. 31