b Institution of Functional Organic Molecules and Materials, School of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng 252059, China;
c Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, Changsha University of Science and Technology, Changsha 410114, China
The controllable construction of different valuable products from the same substrates has become a key goal and significant challenge in modern synthesis [1]. Moreover, the one-pot multibond forming strategy is a versatile platform for selectively and efficiently synthesizing complex molecules as the isolation steps, production costs and chemical waste will be reduced [2]. In this context, radical cyclization of 1, n-dienes has attracted great attention due to its ability to provide rapid access to complex cyclic structures through chemo- and regioselective manners with high efficiency and atom-economy [3].
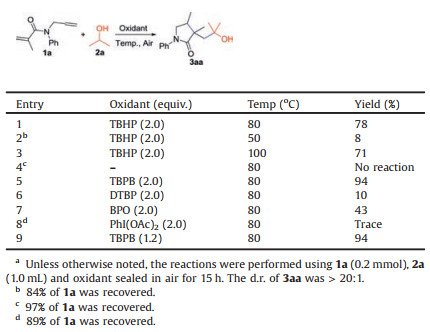
Recently, the direct oxidative α-C(sp3)–H functionalization of alcohols leading to the construction of C—C bonds is emerging as a powerful entry to assemble alcohol skeletons [4]. The group of Guo/Duan [5a] and Liang [5b] independently disclosed interesting oxidative hydroxyalkylarylation of N-arylacrylamides with alcohols, but selective preparation of carbonyl-containing products is limited (Scheme 1a). Lately, Han et al. [5c] reported the radical cyclization of N-allylbenzamides with alcohols using di-tert-butyl peroxide (DTBP) at 120 ℃ (Scheme 1b). However, the high reaction temperature and single product type of this process have limited its application. In2014, a novelworkwas illustratedby Song, Li and coworkers [5d] to achieve1, 2-carboacylationofN-arylacrylamides with alcohols in the presence of Fe(OAc)2 at 110 ℃ (Scheme 1c).However, the radical cyclization of 1, n-dienes initiated by α-C(sp3)–H functionalization of alcohols in a selective mode has yet to be explored so far. In continuation of our interest in green chemistry [6], we wish to report a catalyst-free protocol for the radical cyclization of 1, 6-dienes with alcohols using TBPB as an oxidant at relatively low temperature for the selective preparation of 2- pyrrolidinones (Scheme 1d), which is a crucial class of fivemembered N-heterocycles commonly found in natural products and pharmaceuticals [7]. Notably, this discovery included three significant advances: (1) controllable construction of different products (hydroxy-, carbonyl- and acetal-containing 2-pyrrolidinones) from different alcohols under similar conditions; (2) mild reaction conditions: catalyst-free and relatively low temperature at 80 ℃; (3) the realization of two to four bonds forming in one-pot.
|
Download:
|
| Scheme 1. Direct oxidative α-C(sp3)–H functionalization of alcohols. | |
Our studies began with the reaction of N-allyl-N-phenyl-methacrylamide (1a, 0.2 mmol), propan-2-ol (2a, 1.0 mL) and tert-butyl hydroperoxide (TBHP, 2.0 equiv.) at 80 ℃ sealed in air (Table 1). As expected, the cyclization product 3aa was obtained in 78% yield (entry 1). This serendipity encouraged us to investigate condition screenings to improve the efficiency of this novel cyclization strategy. We first screened the reaction temperature. The outcomes showed that further increase of the temperature had a negative effect on the yield of 3aa, whereas the majority of 1a was recovered at 50 ℃ (entries 2 and 3). Therefore, 80 ℃ was selected as the reaction temperature for further screening. A control experiment indicated that an oxidant was indispensable for this transformation (entry 4). Then the screening of various oxidants, such as TBPB, DTBP, benzoyl peroxide (BPO) and PhI(OAc)2, showed that TBPB was the best choice for delivering 3aa (entries 5–8). Gratifyingly, the oxidant loading could be reduced to 1.2 equiv. without sacrificing chemical yield (94%, entry 9). Thus, we summarized the optimized reaction conditions: 1a (0.2 mmol), 2a (1.0 mL) and TBPB (1.2 equiv.), at 80 ℃ sealed in air for 15 h.
|
|
Table 1 Optimization of reaction conditions.a |
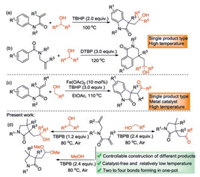
Having the optimized reaction conditions in hand, the scope of this selective cyclization with regard to 1, 6-dienes 1 with secondary alcohols 2 was examined (Scheme 2). First, 1a reacted with cyclopentanol 2b to afford 71% of the target product 3ab. Subsequently, 1, 6-dienes 1b-1e bearing OMe, Me, Cl and CN groups on the N-aromatic ring of 1, 6-dienes were investigated, and the corresponding products 3ba-3ea were obtained in 80%–96% yields. Specifically, the results showed that electronic effect of the substituents on the N-aromatic ring of 1, 6-dienes affected the reaction yields: The N-aromatic ring with electron-donating groups (1b and c) showed higher reactivities than with electron-deficient ones (1d and e). Unfortunately, when benzyl group replaced the aryl substituent on the nitrogen moiety, trace of the expected product 3fa was afforded and 90% of 1f was recovered. Importantly, the reaction of Me substituent at the α-position of alkenyl (R2) with 2a could give the desired product 3ga in 92% yield. 1, 6-Diene bearing the Bn substituent at the α-position of alkenyl (R3) could smoothly be transformed into the corresponding cyclization product 3 ha in 73% yield.
|
Download:
|
| Scheme 2. Selective cyclization of 1, 6-dienes with secondary alcohols. Unless otherwise noted, the reactions were performed using 1 (0.2 mmol), 2 (1.0 mL) and TBPB (1.2 equiv.) at 80 ℃ sealed in air for 15 h. | |
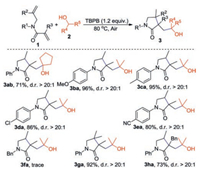
Next, the reaction of 1, 6-dienes with primary alcohols was conducted (Scheme 3). A series of common primary alcohols such as ethanol (2c), n-propanol (2d), n-butanol (2e) and MeOH (2f) were used to react with 1a. Notably, 2c, 2d and 2e reacted smoothly with 1a to afford corresponding products 3ac-ae in 72%–85% yields. To our surprise, 2f could undergo this process and gave the acetal product 3-(2, 2-dimethoxyethyl)-3, 4-dimethyl-1-phenylpyr-rolidin-2-one 5af in 81% yield, but the desired product 4af was not detected. We then chose 2c as representative primary alcohol to react with 1, 6-dienes. Two substituents, namely OMe and CN, on the N-aromatic ring of 1, 6-dienes were well tolerated, affording the products 4bc and 4ec in 87% and 74% yields, respectively. This reaction was compatible with substrate bearing Me substituent at the α-position of alkenyl (R2), leading to the formation of corresponding product 4gc in good yield. Furthermore, 1, 6-dienes bearing the Bn and pH substituents at the α-position of alkenyl (R3) proceeded well to give the desired products 4hc-ic in moderate yields.

|
Download:
|
| Scheme 3. Selective cyclization of 1, 6-dienes with primary alcohols. Unless otherwise noted, the reactions were performed using 1 (0.2 mmol), 2 (1.0 mL) and TBPB (2.4 equiv.) at 80 ℃ sealed in air for 15 h. a 81% of 3-(2, 2-dimethoxyethyl)-3, 4- dimethyl-1-phenylpyrrolidin-2-one (5af) was obtained (d.r. > 20:1). | |
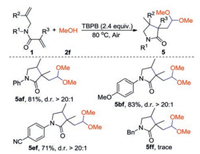
As above mentioned, MeOH (2f) could react with 1a and gave the acetal product 5af in 81% yield, we then investigated the reaction between 2f and 1, 6-dienes (Scheme 4). Substrates with OMe and CN groups on the N-aromatic ring of 1, 6-dienes were easily converted into the desired acetal products 5bf and 5ef in moderate to good yields. However, the reaction of 2f with N-Bn substituted 1, 6-diene 1f gave trace product 5ff.
|
Download:
|
| Scheme 4. Selective cyclization of 1, 6-dienes with MeOH. Unless otherwise noted, the reactions were performed using 1 (0.2 mmol), 2f (1.0 mL) and TBPB (2.4 equiv.) at 80 ℃ sealed in air for 15 h. | |
In order to give a better understanding of this selective cyclization, several control experiments were performed (Scheme 5). Initially, when using 1.2 equiv. of TBPB in the reaction of 1a with 2c, the yield of desired product 4ac was sharply reduced to 41%, and only trace of 4ac' was detected, which indicated that 4ac' was oxidized quickly under this oxidation system (Scheme 5a). Treatment of 1a and butyraldehyde 5a under the standard conditions, but no 4ae was obtained (Scheme 5b). This result implied that 5a was not a possible intermediate and this reaction may be initiated by the α-C(sp3)-H bond functionalization of alcohols. Moreover, we performed two radical trapping experiments with 2.0 equiv. of 2, 2, 6, 6-tetramethylpiperidine-1- oxyl (TEMPO) or 2, 6-di-tert-butyl-4-methylphenol (BHT), but trace of cyclization product 3aa was detected and nearly >90% of 1a was recovered (Scheme 5c). This observation indicated that a radical process might be involved in the current transformation.

|
Download:
|
| Scheme 5. Control experiment. | |
Based on the above experimental results and pertinent work [3b–e, 8, 9], it is assumed that a radical process is involved in the formation of 3, 4 and 5 as shown in Scheme 6. The reaction is initiated by the alkyl radical A, which is caused by the α-C(sp3)–H cleavage of alcohol in the presence of TBPB under heating [8]. Then, the selective radical addition of A to 1a gives intermediate B, which is cyclization with another C=C double bond of 1a to produce intermediate C, followed by hydrogen abstraction from solvent affording product 3 [3b–e, 9]. When R1 = H, the further oxidation of hydroxyl by TBPB generates the 4. More especially, when R2 = H, the acetalization of 4 with MeOH under the current system to form acetal product 5.
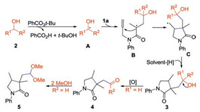
|
Download:
|
| Scheme 6. Possible mechanism for the formation of 3, 4 and 5. | |
In conclusion, a one-pot TBPB promoted approach has been developed for the selective synthesis of hydroxy-, carbonyl- and acetal-containing 2-pyrrolidinones via radical cyclization of 1, 6- dienes with alcohols under catalyst-free conditions. The method exhibits remarkable compatibility with a wide variety of 1, 6- dienes, secondary alcohols and primary alcohols, realizing the controllable construction of different products under similar conditions. Control experiments suggested that this transformation is initiated by the α-C(sp3)-H bond functionalization of alcohols and a radical process is involved. Further application of this radical cyclization is currently ongoing in our laboratory.
Declaration of competing interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AcknowledgementsWe thank the National Natural Science Foundation of China (No. 21801142), the Natural Science Foundation of Zhejiang Province (No. LQ18B020002), the Ningbo Municipal Natural Science Foundation (No. 2019A610021) and the Hunan Provincial Natural Science Foundation of China (No. 2019JJ20008) for financial support.
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2020.04.042.
| [1] |
(a) L. Ping, D.S. Chung, J. Bouffard, S.G. Lee, Chem. Soc. Rev. 46(2017) 4299-4328; (b) J.B. Peng, X.F. Wu, Angew. Chem. Int. Ed. 57(2018) 2-11; (c) J. Mahatthananchai, A.M. Dumas, J.W. Bode, Angew. Chem. Int. Ed. 51(2012) 10954-10990; (d) Y. Wei, M. Shi, ACS Catal. 6(2016) 2515-2524; (e) Z. Cao, Q. Zhu, Y.W. Lin, W.M. He, Chin. Chem. Lett. 30(2019) 2132-2138; (f) K.J. Liu, T.Y. Zeng, J.L. Zeng, et al., Chin. Chem. Lett. 30(2019) 2304-2308; (g) P. Bao, H. Yue, N. Meng, et al., Org. Lett. 21(2019) 7218-7222; (h) P. Bao, N. Meng, Y. Lv, et al., Org. Chem. Front. 6(2019) 3983-3988. |
| [2] |
(a) Y. Hayashi, Chem. Sci. 7(2016) 866-880; (b) J. Sun, Y. Li, Y. Gui, et al., Chin. Chem. Lett. 30(2019) 569-572; (c) H.P. Zhao, G.C. Liang, S.M. Nie, et al., Green Chem. 22(2020) 404-410; (d) H.Y. Bi, M. Du, C.X. Pan, et al., J. Org. Chem. 84(2019) 9859-9868; (e) L.H. Lu, Z. Wang, W. Xia, et al., Chin. Chem. Lett. 30(2019) 1237-1240; (f) Q. Huang, L. Zhu, D. Yi, X. Zhao, W. Wei, Chin. Chem. Lett. 31(2020) 373-376; (g) Y. Zhang, K. Sun, Q. Lv, et al., Chin. Chem. Lett. 30(2019) 1361-1368; (h) C.H. Xu, Y. Li, J.H. Li, J.N. Xiang, W. Deng, Sci. China Chem. 62(2019) 1463-1475; (i) K. Sun, Y.F. Si, X.L. Chen, et al., Adv. Synth. Catal. 361(2019) 4483-4488. |
| [3] |
(a) G.C. Lloyd-Jones, Org. Biomol. Chem. 1(2003) 215-236; (b) Z. Wang, W.-M. He, Chin. J. Org. Chem. 39(2019) 3594-3595; (c) Y. Liu, X.-L. Chen, K. Sun, et al., Org. Lett. 21(2019) 4019-4024; (d) Q.S. Zhao, G.Q. Xu, H. Liang, Z.Y. Wang, P.F. Xu, Org. Lett. 21(2019) 8615-8619; (e) G. Li, Q. Yan, X. Gong, X. Dou, D. Yang, ACS Sustainable Chem. Eng. 7(2019) 14009-14015; (f) Z. Shen, C. Pi, X. Cui, Y. Wu, Chin. Chem. Lett. 30(2019) 1374-1378; (g) X. Mi, Y. Kong, J. Zhang, C. Pi, X. Cui, Chin. Chem. Lett. 30(2019) 2295-2298; (h) Q.Q. Han, G.H. Li, Y.Y. Sun, et al., Tetrahedron Lett. (2020) 151704; (i) K. Sun, X.L. Chen, Y.L. Zhang, et al., Chem. Commun. (Camb.) 55(2019) 12615-12618. |
| [4] |
(a) S.R. Guo, P.S. Kumar, M.H. Yang, Adv. Synth. Catal. 359(2016) 2-25; (b) S.Y. Zhang, F.M. Zhang, Y.Q. Tu, Chem. Soc. Rev. 40(2011) 1937-1949; (c) M.K. Lakshman, P.K. Vuram, Chem. Sci. 8(2017) 5845-5888. |
| [5] |
(a) Y. Meng, L.N. Guo, H. Wang, X.H. Duan, Chem. Commun. (Camb.) 49(2013) 7540-7542; (b) Z.Z. Zhou, H.L. Hua, J.Y. Luo, et al., Tetrahedron 69(2013) 10030-10035; (c) W. Zhou, S.Y. Ni, H.B. Mei, J.L. Han, Y. Pan, Org. Lett. 17(2015) 2724-2727; (d) X.H. Ouyang, R.J. Song, J.H. Li, Eur. J. Org. Chem. 16(2014) 3395-3401. |
| [6] |
(a) Y.N. Meng, Q.Q. Kang, T.T. Cao, et al., ACS Sustainable Chem. Eng. 7(2019) 18738-18743; (b) W.T. Wei, W.W. Ying, W.H. Bao, et al., ACS Sustainable Chem. Eng. 6(2018) 15301-15305; (c) X.D. Xu, T.T. Cao, Y.N. Meng, et al., ACS Sustainable Chem. Eng. 7(2019) 13491-13496; (d) X.X. Meng, Q.Q. Kang, J.Y. Zhang, et al., Green Chem. 22(2020) 1388-1392; (e) W.B. He, L.Q. Gao, X.J. Chen, et al., Chin. Chem. Lett. 31(2020) 1895-1898; (f) W.H. Bao, Z. Wang, X. Tang, et al., Chin. Chem. Lett. 30(2019) 2259-2262; (g) S. Peng, Y.X. Song, J.Y. He, et al., Chin. Chem. Lett. 30(2019) 2287-2290. |
| [7] |
(a) G. Martinez-Ariza, M. Ayaz, S.A. Roberts, et al., Angew. Chem. Int. Ed. 54(2015) 11672-11676; (b) X.L. Du, D.W. Yin, Z.M. Ge, X. Wang, R.T. Li, RSC Adv. 7(2017) 24547-24550; (c) J.C. Anderson, L.R. Horsfall, A.S. Kalogirou, et al., J. Org. Chem. 77(2012) 6186-6198; (d) D. Yang, G.Y. Lian, H.F. Yang, et al., J. Org. Chem. 74(2009) 8610-8615. |
| [8] |
(a) J.H. Fu, J.W. Yuan, Y. Zhang, et al., Org. Chem. Front. 5(2018) 3382-3390; (b) D.Q. Xue, H. Chen, Y.L. Xu, et al., Org. Biomol. Chem.165(2017) 10044-10052. |
| [9] |
X.X. Meng, T.T. Cao, S.Z. Song, et al., Asian J. Org. Chem. 8 (2019) 1827-1829. |
 2020, Vol. 31
2020, Vol. 31